Press release
Duodenoscope Market Segments and Key Trends 2016-2026
Duodenoscope is a side viewing endoscope primarily designed for Endoscopic Retrograde Cholangiopancreatography (ERCP) to diagnose diseases associated with pancreas and bile ducts with the help of fluoroscopic imaging procedure. Duodenoscopes are flexible, light weighted tubes that are relieved through the mouth, throat, and stomach till the duodenum portion. Duodenoscopes are being used in more than 500,000 gastrointestinal procedures in the U.S each year as a minimally invasive way than traditional surgery to drain fluids from biliary and pancreatic ducts which are blocked by cancerous tumors, gallstones or other gastrointestinal conditions.The Duodenoscope is a more complex device than other endoscopes and more tough to clean and disinfect. While these devices play an essential role in the treatment of patients, there is an evidence that some patients have been transmitted with hospital born and other infectious agents, including antibiotic drug-resistant infections. In 2013, the Centers for Disease Control and Prevention (CDC) alerted the FDA regarding possible association of multidrug resistant bacterial infections and duodenoscopes. Even before FDA was reported of the infections by the CDC, FDA was working to reinforce cleaning and disinfection protocols of complex duodenoscopes devices to maximize patient benefit and reduce safety risks. Recently American Society for Gastrointestinal Endoscopy (ASGE) brought together experts in epidemiology, infection control and endoscopy, FDA and CDC representatives, hospitals that practiced epidemics, and device manufacturers. The purposes of these organizations to confirm current FDA and industry guidelines for cleaning duodenoscopes and identify gaps in knowledge and issues to address going onward.
Based on product type, global duodenoscopes market is classified as follows:
Flexible Video Dueodenoscopes
Flexible Non- Video Dueodenoscopes
Request Free Report Sample@ http://www.futuremarketinsights.com/reports/sample/rep-gb-1667
Based on End User type, global duodenoscopes market is classified as follows:
Hospitals
Pediatric Centers
Clinics
Ambulatory Surgical Centers
Currently, owing to extremely spread of infection of Carbapenem-Resistant Enterobacteriaceae (CRE) bacteria, through duodenoscopes, the procedure is probably not performing in an outpatient facility such as clinics and ambulatory surgical centers.
Increasing incidences of pancreatic, bile duct cancer tumors and various gastrointestinal conditions are the major origins contributing to the growth of the global duodenoscopes market. In addition, increasing FDA activities and CDC and manufactures collaboration is in identifying the causes and risk factors for transmission of infectious agents with duodenoscopes and developing new solutions to minimize patient exposure is another major driver, fueling the global duodenoscopes market growth over the forecast period. However, increasing FDA recalls for duodenoscopes owing to challenging cleaning and high-level disinfection procedures associated with complex designed duodenoscopes and rising multidrug-resistant bacterial infections caused by Carbapenem-Resistant Enterobacteriaceae (CRE) such as Klebsiella species and Escherichia coli are major factors are expected to hamper the growth of global duodenoscopes market over the forecast period.
Visit For TOC@ http://www.futuremarketinsights.com/toc/rep-gb-1667
Geographically global duodenoscopes market has been segmented into North America, Latin America, Europe, Asia-Pacific & Japan, Middle East Africa regions. North America region has been estimated as most dominant region in the global duodenoscopes market owing highly developed healthcare infrastructure and high rates of awareness regarding duodenoscopes and its associated infections among physicians. Asia-Pacific & Japan is a lucrative market for global duodenoscopes. Countries in the Asia Pacific regions include, greater China and India together account for largest population pool in the world and thereby have large pool of geriatric population are expected to fuel the growth of duodenoscopes market in the region.
Some of the key companies contributing to global duodenoscopes market are Fujifilm Holdings Corporation, KARL STORZ GmbH & Co. KG., Olympus Corporation, PENTAX Medical Company, and Hoya Corporation.
ABOUT US:
Future Market Insights is the premier provider of market intelligence and consulting services, serving clients in over 150 countries. FMI is headquartered in London, the global financial capital, and has delivery centres in the U.S. and India.
CONTACT:
Future Market Insights
616 Corporate Way,
Suite 2-9018,
Valley Cottage,
New York 10989,
United States
Tel: +1-347-918-3531
Fax: +1-845-579-5705
Email: sales@futuremarketinsights.com
Website: www.futuremarketinsights.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Duodenoscope Market Segments and Key Trends 2016-2026 here
News-ID: 359972 • Views: …
More Releases from Future Market Insights
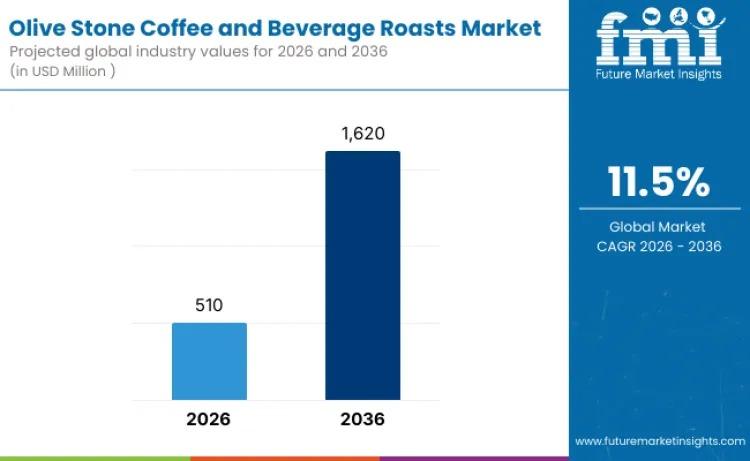
Global Olive Stone Coffee and Beverage Roasts Market to Reach USD 1,620 Million …
The global olive stone coffee and beverage roasts market is entering a high-growth decade, fueled by sustainability innovation and evolving specialty coffee culture. Valued at USD 510 million in 2026, the market is projected to reach USD 1,620 million by 2036, expanding at a compelling CAGR of 11.5%.
As consumers increasingly seek beverages that combine sustainability, functionality, and distinctive taste, olive stone-based roasting solutions are transitioning from niche experimentation to structured…
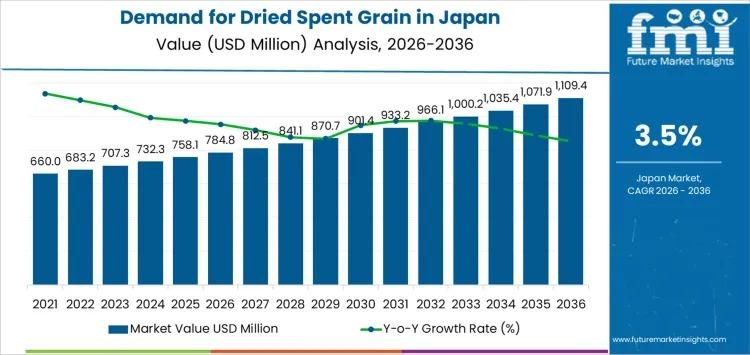
Japan Dried Spent Grain Market to Surpass USD 1.1 Billion by 2036 as Feed Optimi …
Japan's dried spent grain market is entering a decade of steady, value-driven expansion, supported by structured feed demand, brewery byproduct utilization, and rising integration of fiber-rich ingredients into food manufacturing. Industry estimates place the market at USD 784.8 million in 2026, with projections indicating growth to USD 1,109.4 million by 2036, reflecting a CAGR of 3.5%.
Between 2020 and 2026, demand increased from USD 637.5 million to USD 784.8 million, shaped…
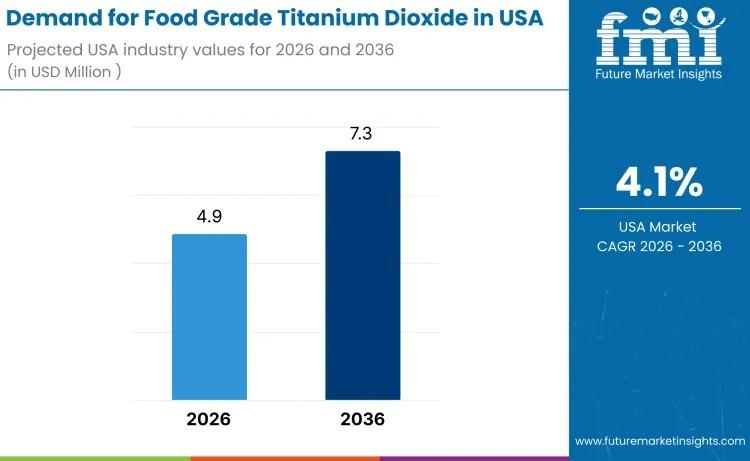
USA Food Grade Titanium Dioxide Market to Reach USD 7.3 Million by 2036 Amid Ste …
The demand for food grade titanium dioxide in the USA is valued at USD 4.9 million in 2026 and is projected to reach USD 7.3 million by 2036, expanding at a CAGR of 4.1%. Growth remains moderate yet stable, supported by continued use of titanium dioxide as a whitening and opacifying agent across confectionery coatings, bakery decorations, sauces, dairy analogues, and processed food matrices.
Despite heightened regulatory scrutiny and evolving clean-label…
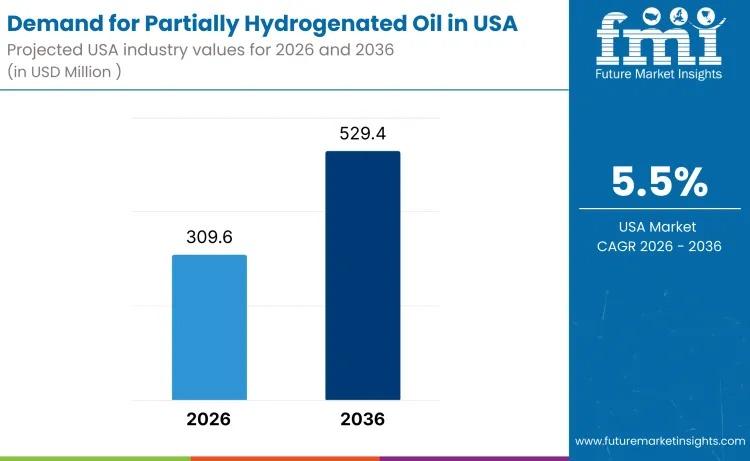
USA Partially Hydrogenated Oil Market to Reach USD 529.4 Million by 2036 Amid Me …
The demand for partially hydrogenated oil in the USA is projected to rise from USD 309.6 million in 2026 to USD 529.4 million by 2036, expanding at a steady CAGR of 5.5%. While edible applications remain tightly regulated, demand persists across specialty industrial and permitted food-related segments where oxidative stability, viscosity control, and texture performance remain critical.
Despite regulatory constraints on trans fats in conventional food manufacturing, PHOs continue to serve…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
