Press release
Rapid Oral Fluid Screening Devices Market Expected to Double to US$ 47.3 Billion by 2033, with a 7.1% CAGR
The global rapid oral fluid screening devices market accounts for a valuation of US$ 23.8 billion in 2023 and is forecasted to climb to US$ 47.3 billion by 2033-end. Fact.MR predicts global demand for rapid oral fluid screening devices to increase at a healthy 7.1% CAGR over the next ten years.The Rapid Oral Fluid Screening Devices Market in the United States has witnessed substantial growth in recent years, owing to a multitude of factors such as increased awareness about drug abuse, stringent regulations, and advancements in technology. These devices have gained prominence as an efficient and non-invasive method to detect the presence of drugs in a person's system, making them essential tools for various industries and organizations. In this article, we will delve into the rapid oral fluid screening devices market in the USA, exploring its growth drivers, challenges, key players, and future prospects.
Get a FREE Sample Copy of Report (Including TOC, List of Tables & Figures, Chart) :https://www.factmr.com/connectus/sample?flag=S&rep_id=2694
Market Drivers
Rising Drug Abuse Concerns: The alarming increase in drug abuse and its adverse effects on public health has prompted government agencies and organizations to adopt strict drug testing measures. Rapid oral fluid screening devices offer a quick and efficient solution to this problem, driving their demand.
Regulatory Mandates: Various regulations have been put in place by federal and state authorities, requiring drug testing in workplaces, schools, and law enforcement agencies. These mandates have been pivotal in propelling the market as organizations seek to comply with the law.
Technological Advancements: Innovations in the design and functionality of rapid oral fluid screening devices have enhanced their accuracy, sensitivity, and ease of use. These advancements have made them more appealing to a wider range of users.
Cost-Efficiency: These devices are cost-effective compared to traditional laboratory tests, making them an attractive option for businesses and institutions looking to manage their budgets while ensuring a drug-free environment.
Convenience: The non-invasive nature of oral fluid testing is a significant advantage, as it eliminates the need for specialized staff or facilities and reduces the discomfort associated with other testing methods.
Challenges
False Positives/Negatives: While rapid oral fluid screening devices have improved in accuracy, they are not infallible. False positives or negatives can occur due to a variety of factors, such as improper usage, cross-reactivity with other substances, or the device's sensitivity.
Privacy Concerns: Collecting oral fluid samples can be seen as invasive by some individuals, raising privacy concerns. Striking a balance between ensuring security and respecting personal privacy remains a challenge.
Adoption Rate: Smaller businesses and organizations may be hesitant to invest in rapid oral fluid screening devices due to the initial cost and training requirements, which can impede widespread adoption.
Legislation and Legal Challenges: The legal landscape regarding drug testing is continuously evolving, leading to potential compliance challenges for businesses and institutions. Ensuring that drug testing policies align with the law can be complex.
Key Players
Abbott Laboratories: Abbott Laboratories offers a range of rapid oral fluid screening devices known for their accuracy and reliability.
Alere (now part of Abbott): Alere, a subsidiary of Abbott, was a pioneer in the development of oral fluid testing solutions.
OraSure Technologies: OraSure Technologies specializes in oral fluid collection devices and is a prominent player in the market.
Quest Diagnostics: Quest Diagnostics provides a comprehensive suite of drug testing services, including rapid oral fluid screening devices.
Roche Diagnostics: Roche Diagnostics offers innovative solutions for oral fluid drug testing, catering to various industries.
Future Prospects
The future of the rapid oral fluid screening devices market in the USA looks promising. Several factors will continue to drive its growth:
Advancements in Technology: Ongoing research and development efforts will likely lead to even more accurate and user-friendly devices, reducing the occurrence of false positives and negatives.
Diverse Applications: The market is expected to expand its applications beyond traditional sectors like workplaces and law enforcement to include areas like healthcare and sports, where regular drug testing is essential.
Telehealth Integration: The integration of oral fluid screening devices into telehealth services could further boost their adoption, making drug testing more accessible.
International Expansion: As other countries face similar drug abuse challenges, US-based companies could expand their reach internationally, driving global market growth.
Legislative Changes: Keeping pace with evolving drug testing regulations will be crucial. Companies that can adapt their products and services to meet changing legal requirements will have a competitive advantage.
In conclusion, the rapid oral fluid screening devices market in the USA is thriving, driven by the pressing need to combat drug abuse, stringent regulations, and technological advancements. While challenges exist, ongoing innovation and expanding applications point to a promising future for this industry. As it continues to evolve, it will play a pivotal role in ensuring safer and more drug-free environments across various sectors in the United States and potentially beyond its borders.
Get Customization on this Report for Specific Research Solutions:https://www.factmr.com/connectus/sample?flag=RC&rep_id=2694
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: sales@factmr.com
About Fact.MR
Fact.MR is a market research and consulting agency with deep expertise in emerging market intelligence. Spanning a wide range - from automotive & industry 4.0 to healthcare, industrial goods to even the most niche categories. 80% of Fortune 1000s trust us in critical decision making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Rapid Oral Fluid Screening Devices Market Expected to Double to US$ 47.3 Billion by 2033, with a 7.1% CAGR here
News-ID: 3573117 • Views: …
More Releases from FactMR
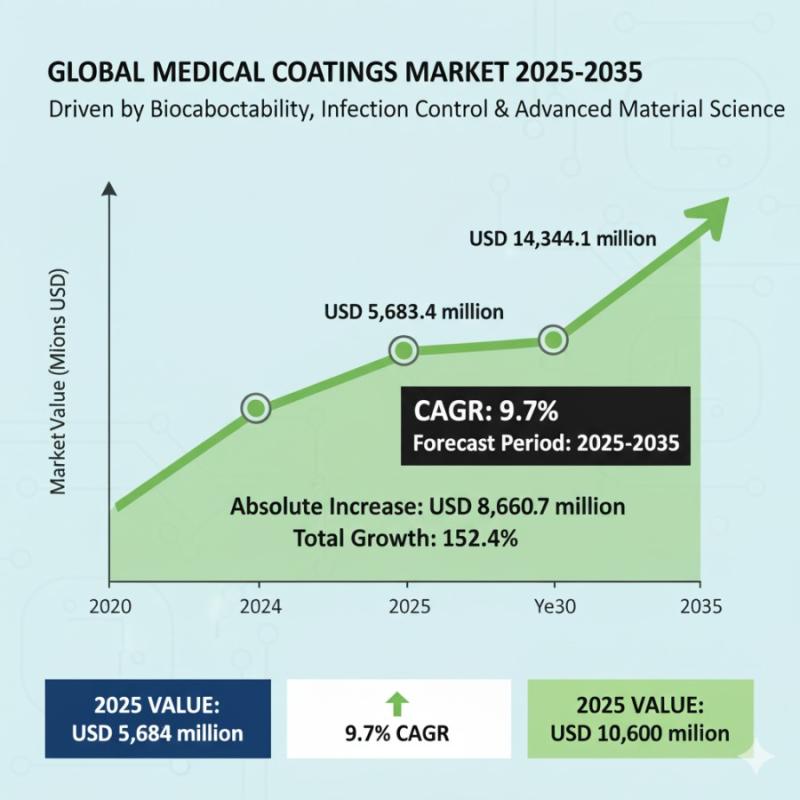
Medical Coatings Market to Hit USD 14,344.1 million by 2035- Growth Accelerates …
The global medical coatings market is set for sustained growth through 2035, powered by minimally invasive procedures, infection prevention priorities, and smart biocompatible innovations. According to Future Market Insights (FMI), the market is valued at USD 5,683.4 million in 2025 and is projected to reach USD 14,344.1 million by 2035, expanding at a compound annual growth rate (CAGR) of 9.7%.
The FMI report, "Medical Coatings Market Size, Share, and Forecast 2025-2035,"…
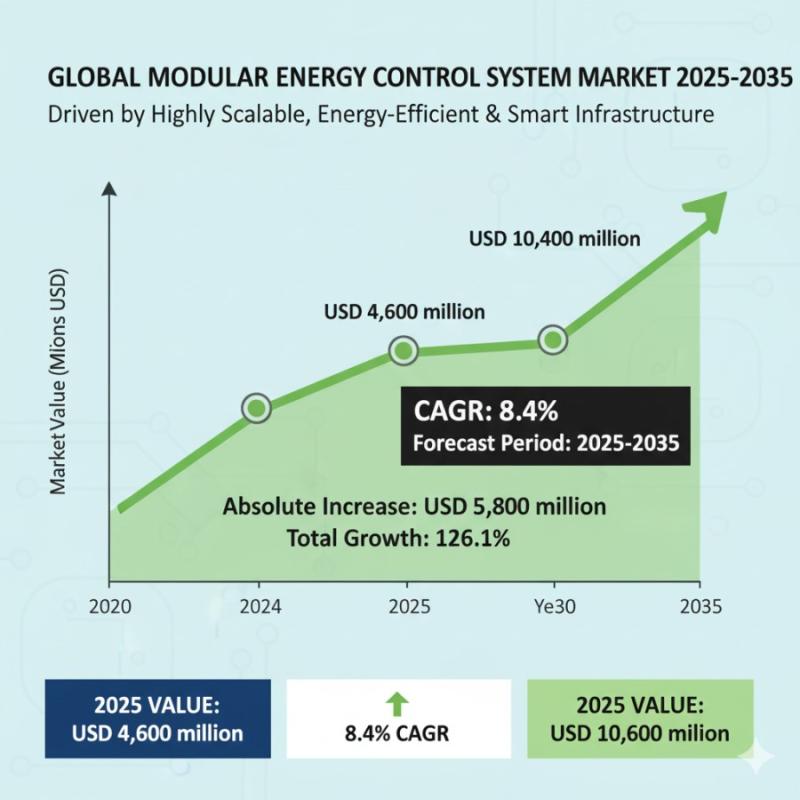
Modular Energy Control System Market to Hit USD 10,400 million by 2035- Growth A …
The global modular energy control system market is set for robust expansion through 2035, fueled by scalable infrastructure, real-time optimization, and seamless renewable energy integration. According to Future Market Insights (FMI), the market is valued at USD 4,600 million in 2025 and is projected to reach USD 10,400 million by 2035, expanding at a compound annual growth rate (CAGR) of 8.4%
The FMI report, "Modular Energy Control System Market Size, Share,…
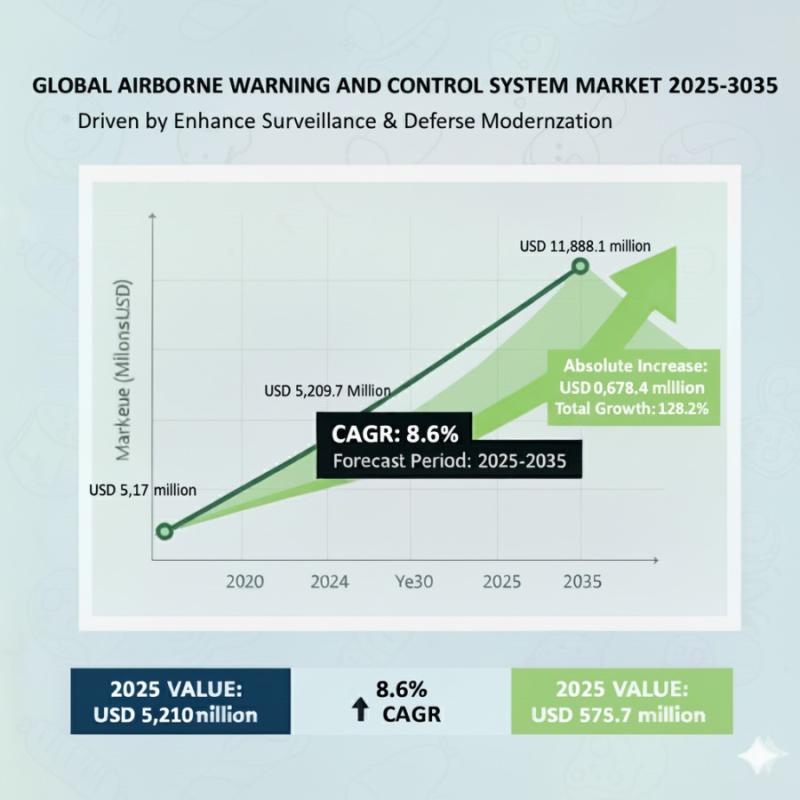
Airborne Warning and Control System Market to Surpass USD 11,888.1 million by 20 …
The global airborne warning and control system (AWACS) market is accelerating toward a decade of robust expansion, driven by escalating geopolitical tensions, defense modernization, and AI-enhanced threat detection. According to Future Market Insights (FMI), the market is valued at USD 5,209.7 million in 2025 and is projected to reach USD 11,888.1 million by 2035, growing at a compound annual growth rate (CAGR) of 8.6%.
The FMI report, "Airborne Warning and Control…
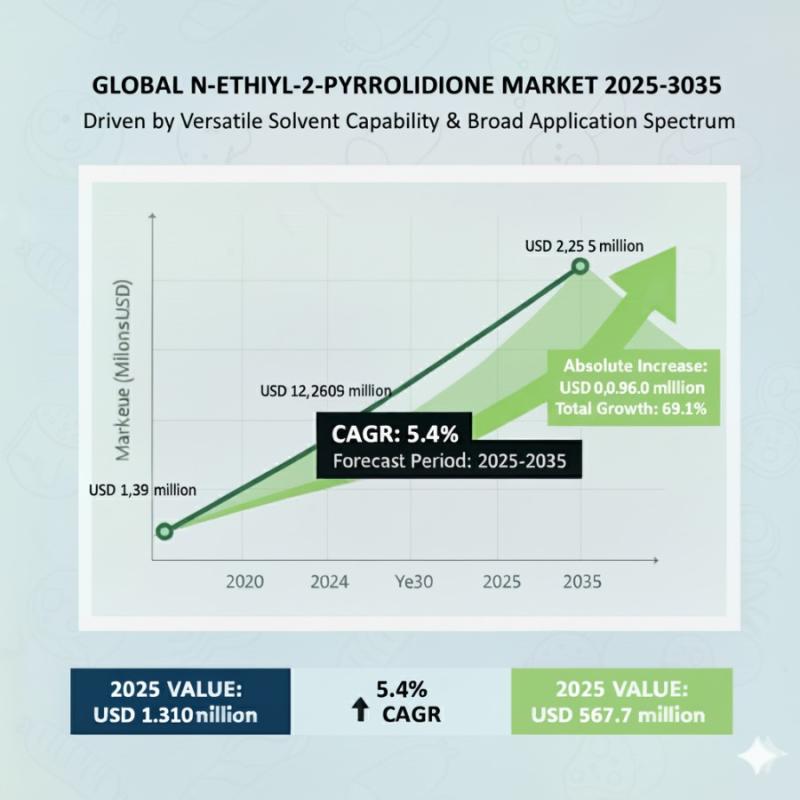
N-Ethyl-2-Pyrrolidone Market to Reach USD 2.35 million by 2035- Steady Growth Le …
The global N-Ethyl-2-Pyrrolidone (NEP) market is poised for consistent expansion through 2035, fueled by rising demand in high-purity electronics, lithium-ion battery production, and pharmaceutical synthesis. According to Future Market Insights (FMI), the market is valued at USD 1.39 million in 2025 and is projected to hit USD 2.35 million by 2035, growing at a compound annual growth rate (CAGR) of 5.4%.
The FMI report, "N-Ethyl-2-Pyrrolidone Market Size, Share, and Forecast 2025-2035,"…
More Releases for Rapid
UYEE Rapid Tooling Announces Expert CNC Machining and Rapid Prototyping Services
UYEE Rapid Tooling Co., Ltd, a trusted provider since 2005, announces its advanced capabilities in high-speed CNC machining, rapid prototyping, and low volume manufacturing solutions.
UYEE Rapid Tooling Co., Ltd, a dedicated leader in manufacturing services since 2005, today highlighted its comprehensive suite of services designed to meet the demanding needs of modern product development and production. With a strong commitment to customer success, UYEE specializes in delivering high-speed CNC machining,…
Rapid Infuser Market - Swift Restoration, Enhanced Recovery: Rapid Infuser Optim …
Newark, New Castle, USA: The "Rapid Infuser Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Rapid Infuser Market: https://www.growthplusreports.com/report/rapid-infuser-market/8896
This latest report researches the industry structure, sales, revenue,…
Rapid Infuser Market - From Emergency to Efficiency: Advancing Patient Outcomes …
Newark, New Castle, USA - new report, titled Rapid Infuser Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Rapid Infuser market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Rapid Infuser market. The report offers an overview of the market, which…
Rapid Pregnancy Tests Market: Size & Trends Shows a Rapid Growth by 2027
The pregnancy test kit confirms pregnancy by detecting the level of human chorionic gonadotropin (HCG) in the urine. The growing demand for an easy and convenient way to get faster results, and the easy availability of pregnancy kits through various distribution channels, including pharmacies/pharmacies, online stores, etc., is increasing the demand for pregnancy test kits.
(Get 15% Discount on Buying this Report)
A full report of Global Rapid Pregnancy Tests Market is…
Rapid Machining Launches New Service
Rapid Machining, one of the largest prototype machining manufacturers in the United States, is excited to announce a new standard 5 day lead time service for lathe parts. Responding to customer demand, Rapid Machining purchased new equipment and reconfigured the manufacturing floor to make this lead-time reduction possible from a standard 7 day lead time. The new service is currently available for lathe parts with a variety of materials and…
RAPID: Lockheed Martin Approved
Nashua, NH – April 4, 2016 – RAPID was announced as an approved vendor for Lockheed Martin as of April 2016. RAPID has been working with Lockheed Martin divisions for a number of years, but the addition to the Approved Vendor list will strengthen and build upon the existing relationship.
Lockheed Martin is an aerospace, defense, security, and advanced technology company that employs over 116,000 professionals worldwide. Lockheed Martin has…
