Press release
Regulatory Information Management Market to Surge at 11.7% CAGR, Reaching $6.05 Billion by 2033
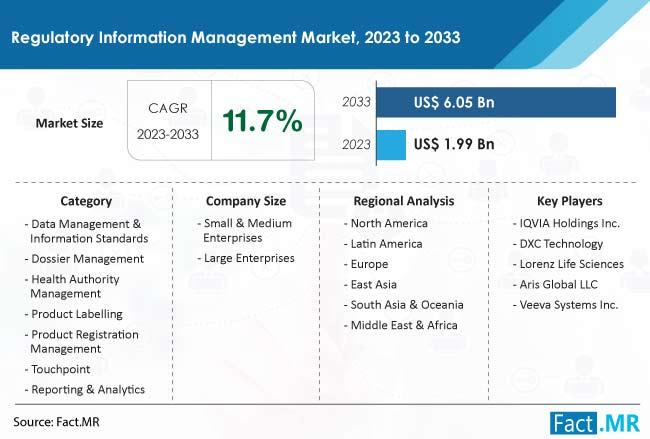
The global Regulatory Information Management Market is projected to grow at a CAGR of 11.7%, reaching a valuation of $6.05 billion by the end of 2033. Regulatory Information Management (RIM) is a modern approach used by regulated industries like pharmaceuticals and medical devices to efficiently handle regulatory information throughout a product's life cycle. Unlike traditional methods, RIM centralizes all regulatory data in one system, streamlining submission management and compliance tracking workflows. This centralized approach ensures real-time collaboration among stakeholders and provides features like version control and audit trails, preventing errors and maintaining accurate regulatory histories.The expansion of this sector is mostly driven by the escalating complexity of global regulatory requirements in industries like pharmaceuticals and healthcare. With an increasing emphasis on electronic submissions and information exchange, organizations are turning to RIM solutions to centralize and streamline regulatory processes, ensuring compliance and accelerating time-to-market. The industry encounters significant challenges due to the constantly changing and diverse nature of regulatory environments across various industries and regions. Many organizations face challenges due to limited budgets and a shortage of skilled personnel. Allocating enough resources for implementing, maintaining, and improving regulatory information management systems is a struggle.
Get Free Sample Copy of This Report-https://www.factmr.com/connectus/sample?flag=S&rep_id=3741
Key Takeaways
In 2023, the North American market is anticipated to capture a significant market share of 35%, underscoring its dominance in the Regulatory Information Management sector. This growth is particularly evident in the United States, where the market is poised to advance rapidly due to a heightened emphasis on optimizing the drug development pipeline. The U.S. pharmaceutical industry's focus on efficiency and innovation in regulatory processes has driven substantial investments in regulatory information management systems, ensuring compliance and streamlining operations. This proactive approach not only accelerates drug development timelines but also enhances the overall quality and safety of pharmaceutical products, making the U.S. a critical hub for regulatory advancements and technological integration in the healthcare sector.
Meanwhile, the pharmaceutical market in China is experiencing rapid expansion, positioning the region as a highly profitable market. This growth is fueled by a surge in pharmaceutical manufacturing activities, driven by the country's strategic investments in healthcare infrastructure and innovation. China's commitment to bolstering its pharmaceutical sector is reflected in the increasing adoption of advanced regulatory information management systems, which are crucial for maintaining compliance with international standards and facilitating global market access. As a result, China is emerging as a key player in the global pharmaceutical landscape, with its robust manufacturing capabilities and regulatory advancements contributing to its growing prominence and profitability in the industry.
List of Key Companies Profiled in The Report
DXC Technology
Ennov SA
Sparta Systems Inc.
Extedo GmbH
NNIT A/S
Lorenz Life Sciences
Dovel Technologies Inc.
PAREXEL International
IQVIA Holdings Inc.
Others
Market Trends
As industries grapple with evolving regulations and compliance challenges, the regulatory information management market is subject to dynamic trends that shape its applications, tools, and user expectations. This part of the article explores the latest trends, including the increasing use of automation in regulatory processes, the rise of real-time monitoring solutions, and the integration of data analytics for proactive compliance management. Recognizing and adapting to these trends are essential for businesses aiming to stay ahead in the competitive landscape of the regulatory information management market.
Winning strategies
It is crucial for major players in the industry to prioritize smooth integration with regulatory databases, ensuring their platforms offer immediate access to the most up-to-date regulatory information and updates.
A successful strategy involves creating customized solutions tailored to the unique regulatory challenges in the life sciences and healthcare sectors. This strategy includes designing platforms with features specifically crafted to ensure compliance with industry-specific regulations.
It's important for leading companies to create adaptable solutions that suit the needs and budgets of small and medium-sized enterprises (SMEs). These tailored solutions allow SMEs to meet regulatory requirements without requiring extensive resources.
Want Full Report? Enquire Here-https://www.factmr.com/report/3741/regulatory-information-management-market
Market Competition
The regulatory information management market comprises key suppliers that are concentrating on the introduction of innovative products equipped with advanced features to enhance their global sales and revenue generation capabilities. Key players in the market are DXC Technology, Ennov SA, Sparta Systems Inc., Extedo GmbH, NNIT A/S, Lorenz Life Sciences, Dovel Technologies Inc., PAREXEL International, and IQVIA Holdings Inc
In February 2023, ArisGlobal, a well-known player in the life sciences sector, unveiled a new product designed to assist medical device providers in navigating the investigational phases of drug development.
In August 2023, Boyds, a worldwide drug development consultancy, revealed the introduction of its new regulatory operations service, aiming to assist clients throughout the entire lifecycle of their drug development projects. The service is entirely conducted in-house, and the company utilizes LORENZ docuBridge, a widely used software for regulatory information management.
Contact:
US Sales Office
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583, +353-1-4434-232
Email: sales@factmr.com
About Fact.MR:
Fact.MR is a market research and consulting agency with deep expertise in emerging market intelligence. Spanning a wide range - from automotive & industry 4.0 to healthcare, technology, chemical and materials, to even the most niche categories.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Information Management Market to Surge at 11.7% CAGR, Reaching $6.05 Billion by 2033 here
News-ID: 3538399 • Views: …
More Releases from Fact.MR
Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
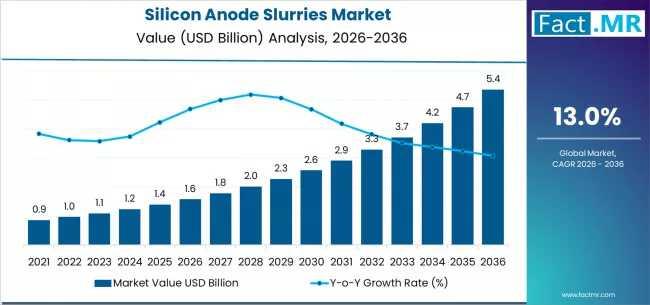
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…

Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…
Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
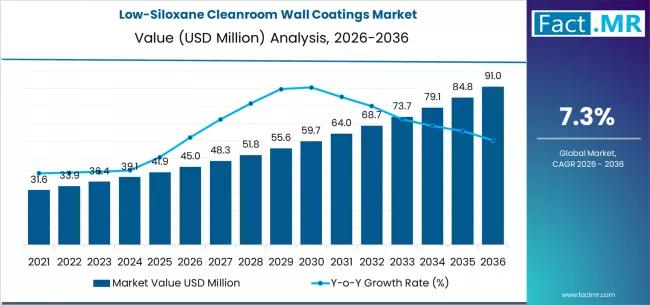
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…

Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…