Press release
Spaulding Clinical to Disrupt Cardiac Safety Industry with Latest Innovation at DIA 2016
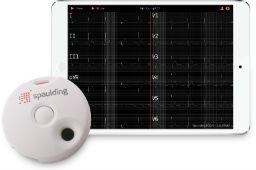
Wireless, hand-held electrocardiograph designed to simplify the collection and transmission of ECG data for Pharmaceutical SponsorsJune 23, 2016 - West Bend, Wisconsin, USA - Spaulding Clinical Research, LLC, a global clinical pharmacology, cardiac safety and biometrics solutions provider, today announced that the Spaulding Electrocardiograph 2100iQ™ is to be exhibited at the Drug Information Association’s (DIA) 2016 conference.
The Spaulding 12-Lead ECG product line was originally launched in 2011 and is now in use in over 35 countries. The Spaulding Electrocardiograph 2100iQ has been optimized to visualize collected real-time digital ECG data via Bluetooth® using iOS™ 7+, Android™ 4.2+ and Windows™ 7+ devices. Data is wirelessly uploaded to the Spaulding webECG™ management cloud for Cardiologist over-reads and is designed to integrate with electronic medical records and clinical information management systems.
Features:
Small (3.5 in), Lightweight (3.3 oz) Design for Easy Storage and Reduced Shipping Costs
Single Button Operation for Ease of Use
Protocol-Driven User Interface
Multiple Protocol Support to Eliminate Need of Additional Equipment
Stores up to 5 minutes of ECG data, Eliminates Need for Triplicate ECGs
“The Spaulding Electrocardiograph 2100iQ makes it logistically and financially feasible to collect very high-quality, centralized ECG data,” said Dr. Jay Mason, Chief Medical Officer. “This innovation in cardiac safety sets a new standard for data collection and gives Pharmaceutical Sponsors the ability to make earlier and better-informed drug development decisions.”
About Spaulding Clinical Research, LLC
Spaulding Clinical Research, LLC is a global CRO providing Phase I - IV drug development services to the biotechnology and pharmaceutical industries. Spaulding Clinical Research operates a 200-bed Clinical Pharmacology Unit, Cardiac Core Laboratory and provides full Biometrics/Scientific Affairs services.
Spaulding Clinical Research, LLC
525 S. Silverbrook Drive
West Bend, WI 53095
Tyler C. Borst
Manager of Marketing
262.334.6020
info@spauldingclinical.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Spaulding Clinical to Disrupt Cardiac Safety Industry with Latest Innovation at DIA 2016 here
News-ID: 345866 • Views: …
More Releases from Spaulding Clinical Research, LLC

Spaulding Clinical Research Named “Best Phase I Drug Development CRO”
Spaulding Clinical Research, LLC, a global clinical pharmacology, cardiac safety and biometrics solutions provider, has been recognized as the Best Phase I Drug Development CRO in the 2016 International Life Sciences Awards. Spaulding Clinical Research was selected by an expert panel and based on customer reviews, capabilities, previous accolades and overall company performance.
The International Life Sciences Awards, sponsored by Global Health & Pharma (GHP) Magazine, “recognize the exceptional work of…

Spaulding Clinical Research Announces Addition to Cardiac Core Lab Management Te …
Shane Braninburg Joins Cardiac Safety Solutions Team to Support Growth Initiatives
June 07, 2016 - West Bend, Wisconsin, USA - Spaulding Clinical Research, LLC, a global clinical pharmacology, cardiac safety and biometrics solutions provider, today announced the addition of Shane Braninburg as Senior Director of Core Lab Services. Mr. Braninburg brings more than 15 years of global business expertise to Spaulding, including extensive operational and management experience…

Spaulding Clinical Receives FDA 510(k) Clearance for 12-Lead ECG Device
Wireless, hand-held electrocardiograph designed to streamline the collection and transmission of ECG data for doctors and patients.
May 24, 2016 - West Bend, Wisconsin, USA - Spaulding Medical, LLC, a wholly owned subsidiary of Spaulding Clinical Research, LLC and provider of cardiac safety solutions, today announced the FDA 510(k) Clearance for their newest 12-Lead ECG device: the Spaulding Electrocardiograph 2100iQ™.
The Spaulding 12-Lead ECG product line was originally launched in 2011 and…
More Releases for Electrocardiograph
Mobile Electrocardiograph Monitor Market Size Analysis by Application, Type, and …
USA, New Jersey- According to Market Research Intellect, the global Mobile Electrocardiograph Monitor market in the Internet, Communication and Technology category is projected to witness significant growth from 2025 to 2032. Market dynamics, technological advancements, and evolving consumer demand are expected to drive expansion during this period.
The market for mobile electrocardiographs (ECG) monitors is expanding quickly as a result of the growing need for remote patient monitoring and the prevalence…
Key Influencer in the Electrocardiograph (ECG) Market 2025: Surge In Cardiovascu …
How Are the key drivers contributing to the expansion of the electrocardiograph (ecg) market?
An increase in cardiovascular disease incidents is predicted to advance the electrocardiograph (ECG) market. Cardiovascular ailments comprise a set of disorders impacting the heart and blood vessels, including coronary artery disease, heart failure, arrhythmias, and heart valve issues. ECGs play a crucial role in the diagnosis, treatment, and management of these diseases, helping physicians assess the electrical…
Wireless Electrocardiograph Market Size 2024 to 2031.
Market Overview and Report Coverage
A Wireless Electrocardiograph (ECG) is a portable device used for monitoring and recording the electrical activity of the heart. It allows for continuous monitoring of a patient's heart rate and rhythm without the need for wires or cables.
The current outlook for the Wireless Electrocardiograph Market is positive, with increasing adoption of remote patient monitoring devices and advancements in wireless technology driving growth. The market…
Global Electrocardiograph (ECG) Market Research Report
This report studies the global Electrocardiograph (ECG) market status and forecast, categorizes the global Electrocardiograph (ECG) market size (value & volume) by manufacturers, type, application, and region. This report focuses on the top manufacturers in North America, Europe, Japan, China, and other regions (India, Southeast Asia). The major manufacturers covered in this report GE Healthcare Philips BioTelemetry Suzuken Fukuda Denshi Welch Allyn Mortara Instrument NIHON KOHDEN Spacelabs Healthcare Mindray Medical…
Top Investment Pockets in Electrocardiograph (ECG) Industry
Electrocardiograph market was valued at $4,516 million in 2016, and is projected to reach $6,637 million by 2023, growing at a CAGR of 5.6% from 2017 to 2023. The 3-6 lead type ECG accounted for four-ninths share of the global market in 2016.
Get PDF sample copy: https://www.alliedmarketresearch.com/request-sample/3372
Recent technological advancements in ECG devices with enhanced features such as mobile ECG devices with a wireless system that can transmit data to monitoring…
Gastrointestinal Electrocardiograph Market Trends and Opportunities 2023
Gastrointestinal diseases are mild to chronic disorders related to gastrointestinal tract (GIT), which includes diseases related to esophagus, stomach, intestines, liver, pancreas and other associated body organs. Gastrointestinal diseases may or may not be life-threatening. Gastrointestinal disorders are usually chronic in nature and cause severe inflammation and irritation of bowel.
Read the Comprehensive Overview of Gastrointestinal Electrocardiograph Market: http://www.transparencymarketresearch.com/gastrointestinal-electrocardiograph-market.html
A large number of diagnostic tests and tools are used in the…