Press release
Spaulding Clinical Receives FDA 510(k) Clearance for 12-Lead ECG Device
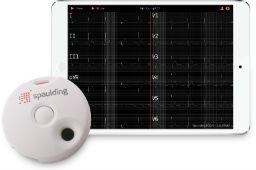
Wireless, hand-held electrocardiograph designed to streamline the collection and transmission of ECG data for doctors and patients.May 24, 2016 - West Bend, Wisconsin, USA - Spaulding Medical, LLC, a wholly owned subsidiary of Spaulding Clinical Research, LLC and provider of cardiac safety solutions, today announced the FDA 510(k) Clearance for their newest 12-Lead ECG device: the Spaulding Electrocardiograph 2100iQ™.
The Spaulding 12-Lead ECG product line was originally launched in 2011 and is now in use in over 35 countries.
The Spaulding Electrocardiograph 2100iQ has been optimized to visualize collected real-time digital ECG data via BluetoothⓇ using iOS™ 7+, Android™ 4.2+ and Windows™ 7+ devices. Data is wirelessly uploaded to the Spaulding webECG™ management cloud for Cardiologist over-reads and is designed to integrate with electronic medical records and clinical information management systems.
“The Spaulding Electrocardiograph 2100iQ is the tangible culmination of our mission to deliver high-quality data in less time and at a reduced cost,” said Randol Spaulding, CEO of Spaulding Clinical. “With our FDA 510(k) Clearance in place, we’ve reinforced our commitment to being an innovation leader in cardiac safety by providing patients, partners and physicians with a mobile ECG solution our competitors cannot.”
About Spaulding Medical
Spaulding Medical, LLC is a wholly owned subsidiary of Spaulding Clinical Research, LLC. Spaulding Medical is where innovative engineering meets purposeful design, ushering in a new paradigm in cardiac safety. The Mission: to make hospital quality cardiac care affordable and accessible by leveraging 150 collective years of cardiac safety expertise into viable health solutions.
Spaulding Clinical Research, LLC
525 S. Silverbrook Drive, West Bend, WI 53095
Tyler C. Borst, Manager of Marketing
info@spauldingclinical.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Spaulding Clinical Receives FDA 510(k) Clearance for 12-Lead ECG Device here
News-ID: 343035 • Views: …
More Releases from Spaulding Clinical Research, LLC

Spaulding Clinical Research Named “Best Phase I Drug Development CRO”
Spaulding Clinical Research, LLC, a global clinical pharmacology, cardiac safety and biometrics solutions provider, has been recognized as the Best Phase I Drug Development CRO in the 2016 International Life Sciences Awards. Spaulding Clinical Research was selected by an expert panel and based on customer reviews, capabilities, previous accolades and overall company performance.
The International Life Sciences Awards, sponsored by Global Health & Pharma (GHP) Magazine, “recognize the exceptional work of…
Spaulding Clinical to Disrupt Cardiac Safety Industry with Latest Innovation at …
Wireless, hand-held electrocardiograph designed to simplify the collection and transmission of ECG data for Pharmaceutical Sponsors
June 23, 2016 - West Bend, Wisconsin, USA - Spaulding Clinical Research, LLC, a global clinical pharmacology, cardiac safety and biometrics solutions provider, today announced that the Spaulding Electrocardiograph 2100iQ™ is to be exhibited at the Drug Information Association’s (DIA) 2016 conference.
The Spaulding 12-Lead ECG product line was originally launched in…

Spaulding Clinical Research Announces Addition to Cardiac Core Lab Management Te …
Shane Braninburg Joins Cardiac Safety Solutions Team to Support Growth Initiatives
June 07, 2016 - West Bend, Wisconsin, USA - Spaulding Clinical Research, LLC, a global clinical pharmacology, cardiac safety and biometrics solutions provider, today announced the addition of Shane Braninburg as Senior Director of Core Lab Services. Mr. Braninburg brings more than 15 years of global business expertise to Spaulding, including extensive operational and management experience…
More Releases for ECG
ECG Cable and ECG Lead Wires Market
Allied Market Research (USA, Oregon, Portland) Published Latest Report titled, ‘ECG Cable and ECG Lead Wires Market: Global Opportunity Analysis and Industry Forecast 2020-2027’.
This market research study determines the increase in changes and the aspects which are likely to have an impact on the growth of the ECG Cable and ECG Lead Wires Market. Increased demand for the technologies is also one of the factors, which are likely to boost…
ECG Devices Market: Resting ECG Rises in Popularity; Multi-purpose Devices Lever …
Cardiac disease has been one of the primary causes of catastrophic mortality. According to American Heart Association, cardiovascular diseases are responsible for taking more lives each year than all types of cancer and chronic lower respiratory disease combined. Detecting the symptoms of cardiac disease, ergo, becomes imperative. The uses of ECG devices, as such, to diagnose and assist in treating some types of heart disease and arrhythmias, showcase trends or…
ECG Cable and ECG Lead Wires Market Opportunity Analysis, 2018-2026
Electrocardiograph machines are used to measure the electrical activity of a patient’s heart. The electrical activity of the heart is recorded with the help of 12-lead ECGs and 10 electrodes placed on patient’s limbs and surface of the chest. Furthermore, ECG applies to monitor the patient’s emergency situation to obtain quick results of heart’s size, position of heart chambers, the rate of the heart, rhythm, and any damage to heart…
China ECG Cable and ECG Lead Wires Market Research Report 2017 | MRH
Market Research Hub (MRH) has recently published the latest market study to its online portal, which is titled as “China ECG Cable and ECG Lead Wires Market Research Report 2017” This study offers professional analysis of the current state of ECG Cable and ECG Lead Wires Market.
Request Free Sample Report@ http://www.marketresearchhub.com/enquiry.php?type=S&repid=948044
This report studies ECG Cable and ECG Lead Wires in China market, focuses on the top players in China…
ECG Systems Market grows with rising demand for ECG systems from emerging countr …
Global ECG Systems Market
Electrocardiography (ECG) is a way of measuring the electrical activity of the heart. During each heartbeat, cardiac muscles undergo depolarization, creating a small electrical charge. This charge, the frequency and magnitude of which serves as a sign of heart health, is detected via electrodes on the chest and limbs. ECG systems allow cardiologists to diagnose several cardiac conditions, such as arrhythmias, congenital cardiac problems, and poor blood…
ECG Cables and ECG Lead Wires Market 2015-2022 Research Report - DecisionDatabas …
The new research report on ECG Cables and ECG Lead Wires Market offered by DecisionDatabases.com provides Global Industry Analysis, Size, Share, Growth, Trends and Forecast Till 2022.
Get FREE Sample Report Copy @ http://www.decisiondatabases.com/contact/download-sample-14935
The report on global ECG cables and ECG lead wires market evaluates the growth trends of the industry through historical study and estimates future prospects based on comprehensive research. The report extensively provides the market share, growth, trends…
