Press release
Regulatory Affairs Outsourcing Market Is Estimated To Secure A Value Of US$ 15.11 Billion By 2032
The global regulatory affairs outsourcing market is estimated to secure a market value of US$ 15.11 Bn by 2032. The worth of the market is anticipated to be US$ 7 Bn in 2022, and the projected growth rate is 8% during the forecast period from 2022 to 2032.According to Fact. MR- a market research and competitive intelligence provider- demand for regulatory affairs outsourcing expanded at a value CAGR of 6.5% from 2015 to 2021, reaching a value of US$ 6 Bn. Regulatory affairs outsourcing services are widely used to develop generic and orphan drugs, which acts as a catalyst to market growth.
𝗗𝗼𝘄𝗻𝗹𝗼𝗮𝗱 𝗙𝗿𝗲𝗲 𝗦𝗮𝗺𝗽𝗹𝗲 𝗖𝗼𝗽𝘆 𝗼𝗳 𝘁𝗵𝗶𝘀 𝗥𝗲𝗽𝗼𝗿𝘁- https://www.factmr.com/connectus/sample?flag=S&rep_id=7143?PJ
𝗖𝘂𝗿𝗿𝗲𝗻𝘁 𝗠𝗮𝗿𝗸𝗲𝘁 𝗧𝗿𝗲𝗻𝗱𝘀:
Complex Regulatory Landscape: The pharmaceutical and healthcare industries face an increasingly intricate regulatory landscape, characterized by evolving guidelines and global harmonization efforts. Navigating this complexity requires specialized expertise, prompting companies to turn to outsourcing partners.
Time-to-Market Pressures: The competitive nature of the life sciences industry demands swift product development and market entry. Regulatory affairs outsourcing allows companies to accelerate their regulatory submissions and approvals, reducing time-to-market.
Cost-Efficiency: Outsourcing regulatory affairs services can be more cost-effective than maintaining an in-house regulatory team. Companies can avoid the overhead costs associated with hiring, training, and retaining regulatory professionals.
Global Expansion: As life sciences companies expand into international markets, they must comply with various regulatory requirements. Regulatory affairs outsourcing providers offer global expertise, helping companies penetrate new markets more effectively.
Specialized Expertise: Regulatory affairs service providers are often staffed with specialized professionals who are well-versed in specific areas, such as medical devices, pharmaceuticals, or biotechnology. This expertise is valuable in navigating industry-specific regulations.
𝗞𝗲𝘆 𝗠𝗮𝗿𝗸𝗲𝘁 𝗦𝗲𝗴𝗺𝗲𝗻𝘁𝘀 𝗖𝗼𝘃𝗲𝗿𝗲𝗱 𝗶𝗻 𝘁𝗵𝗲 𝗚𝗹𝗼𝗯𝗮𝗹 𝗥𝗲𝗴𝘂𝗹𝗮𝘁𝗼𝗿𝘆 𝗔𝗳𝗳𝗮𝗶𝗿𝘀 𝗢𝘂𝘁𝘀𝗼𝘂𝗿𝗰𝗶𝗻𝗴 𝗠𝗮𝗿𝗸𝗲𝘁
𝗕𝘆 𝗦𝗲𝗿𝘃𝗶𝗰𝗲𝘀
Regulatory Consulting Outsourcing
Legal Representation Outsourcing
Regulatory Writing & Publishing Outsourcing
Other Regulatory Affairs Outsourcing Services
𝗕𝘆 𝗖𝗼𝗺𝗽𝗮𝗻𝘆 𝗦𝗶𝘇𝗲
Small Sized Companies
Medium Sized Companies
Large Sized Companies
𝗕𝘆 𝗖𝗮𝘁𝗲𝗴𝗼𝗿𝘆
Drugs
Generics
Innovators
Biologics
Biotech
ATMPs
Biosimilars
Medical Devices
Therapeutic
Diagnostic
𝗕𝘆 𝗜𝗻𝗱𝗶𝗰𝗮𝘁𝗶𝗼𝗻
Oncology
Neurology
Radiology
Immunology
Other Indications
𝗕𝘆 𝗦𝘁𝗮𝗴𝗲
Pre-Clinical
Clinical
Post Market Authorization
𝗕𝘆 𝗘𝗻𝗱 𝗨𝘀𝗲
Medical Device Companies
Pharmaceutical Companies
Biotechnology Companies
𝗥𝗲𝗴𝗶𝗼𝗻𝘀
North America
MEA
Latin America
Europe
Asia-Pacific
𝗞𝗲𝘆 𝗧𝗮𝗸𝗲𝗮𝘄𝗮𝘆𝘀 𝗳𝗿𝗼𝗺 𝘁𝗵𝗲 𝗠𝗮𝗿𝗸𝗲𝘁 𝗦𝘁𝘂𝗱𝘆
The global regulatory affairs outsourcing market to value US$ 7 Bn in 2022
From 2015-2021, regulatory affairs outsourcing demand flourished at a 6.5% CAGR
APAC to garner a market share of 37% in 2022 with respect to regulatory affairs outsourcing
Clinical studies segment procured the largest market share of 46% in 2021.
Based on end-user segment, the pharmaceutical companies segment to secure the largest market share of 38% in 2022.
𝗞𝗲𝘆 𝗣𝗹𝗮𝘆𝗲𝗿𝘀 𝗼𝗳 𝘁𝗵𝗲 𝗚𝗹𝗼𝗯𝗮𝗹 𝗖𝗹𝗼𝘂𝗱 𝗖𝗼𝗺𝗽𝘂𝘁𝗶𝗻𝗴 𝗠𝗮𝗿𝗸𝗲𝘁
Accell Clinical Research LLC
GenPact Ltd.
Criterium Inc.
PRA Health Sciences
Promedica International
WuxI AppTec Inc.
Medspace
Charles River Laboratories International Inc.
ICON Plc.
Labcorp
𝗖𝗼𝗺𝗽𝗲𝘁𝗶𝘁𝗶𝘃𝗲 𝗟𝗮𝗻𝗱𝘀𝗰𝗮𝗽𝗲
The players of the global regulatory affairs outsourcing market focus on expanding their global influence. Some of the adopted strategies are partnerships, collaborations, and acquisitions. Recent key developments among key players are:
ProPharma Group, an Odyssey Investment Partners portfolio company, announced the acquisition of iSafety Systems in August 2021. Pharmacovigilance outsourcing company iSafety is based in India.
ICON plc bought PRA Health Sciences, a CRO, in July 2021. The acquisition was made with the intention of expanding ICON plc's offering of services. ICON will remain the company's official name. The organization seeks to become the most advanced clinical research organization and healthcare intelligence by bringing together 38,000 workers from 47 different nations.
𝗜𝗻𝗱𝘂𝘀𝘁𝗿𝘆 𝗡𝗲𝘄𝘀 𝗮𝗻𝗱 𝗗𝗲𝘃𝗲𝗹𝗼𝗽𝗺𝗲𝗻𝘁𝘀:
Technological Advancements: Regulatory affairs outsourcing is benefiting from advancements in technology, such as regulatory information management (RIM) systems and artificial intelligence (AI) tools that enhance data management and submission processes.
Data Security and Compliance: Providers are continually enhancing data security and compliance measures to protect sensitive information and maintain adherence to data privacy regulations like GDPR.
Diversification of Services: Outsourcing firms are expanding their service portfolios to include not only regulatory submissions but also pharmacovigilance, quality assurance, and other related functions to offer comprehensive regulatory solutions.
Global Regulatory Network: Providers are establishing a global network to facilitate efficient communication with health authorities worldwide, streamlining the regulatory approval process for their clients.
Cross-Functional Collaboration: Collaboration with other service providers in the life sciences ecosystem, such as contract research organizations (CROs) and clinical research organizations (CROs), is increasing to provide integrated solutions that encompass multiple aspects of product development and regulatory approval.
𝗚𝗲𝘁 𝗖𝘂𝘀𝘁𝗼𝗺𝗶𝘇𝗮𝘁𝗶𝗼𝗻 𝗼𝗻 𝘁𝗵𝗶𝘀 𝗥𝗲𝗽𝗼𝗿𝘁 𝗳𝗼𝗿 𝗦𝗽𝗲𝗰𝗶𝗳𝗶𝗰 𝗥𝗲𝘀𝗲𝗮𝗿𝗰𝗵 𝗦𝗼𝗹𝘂𝘁𝗶𝗼𝗻𝘀:https://www.factmr.com/connectus/sample?flag=RC&rep_id=7143?PJ
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E Mail : sales@factmr.com
Fact.MR is a market research and consulting agency with deep expertise in emerging market intelligence. Spanning a wide range - from automotive & industry 4.0 to healthcare, technology, chemical and materials, to even the most niche categories.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Affairs Outsourcing Market Is Estimated To Secure A Value Of US$ 15.11 Billion By 2032 here
News-ID: 3266884 • Views: …
More Releases from Fact.MR

Citrus Fiber Market is Expanding at a 5.7% of CAGR by 2034 | Fact.MR Report
The global Citrus Fiber Market is projected to experience substantial growth over the next decade, driven by rising demand for clean-label ingredients, functional food components, and sustainable fiber sources.
Market analysts estimate that the market, valued at approximately USD 350 million in 2025, is expected to reach around USD 720 million by 2035, expanding at a compound annual growth rate (CAGR) of about 7.5% during the forecast period.
Get Access of…
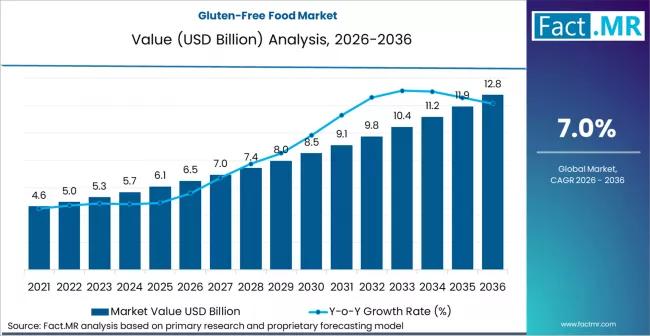
Gluten-Free Food Market is Predicted to Grow to USD 6.5 Billion in 2026 and USD …
The global gluten-free food market is entering a phase of mainstream consolidation, projected to grow from a valuation of USD 7.4 billion in 2026 to approximately USD 15.2 billion by 2036. This represents a steady compound annual growth rate (CAGR) of 7.5% over the ten-year forecast period.
While initially driven by medical necessity for celiac disease patients, the market is now being propelled by "lifestyle consumers" who perceive gluten-free products…
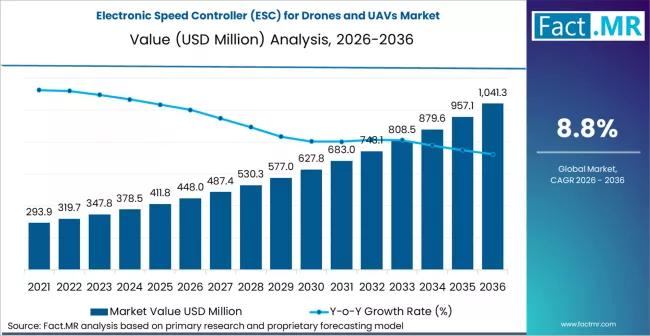
Electronic Speed Controller for Drones and UAVs Market is Valued USD 448.0 milli …
The global electronic speed controller (ESC) for drones and UAVs market is experiencing a rapid technological surge, projected to grow from a valuation of USD 1.8 billion in 2026 to approximately USD 5.1 billion by 2036. This represents a strong compound annual growth rate (CAGR) of 11.0% over the ten-year forecast period.
The market is being propelled by the proliferation of long-endurance commercial drones, the militarization of small FPV (First…
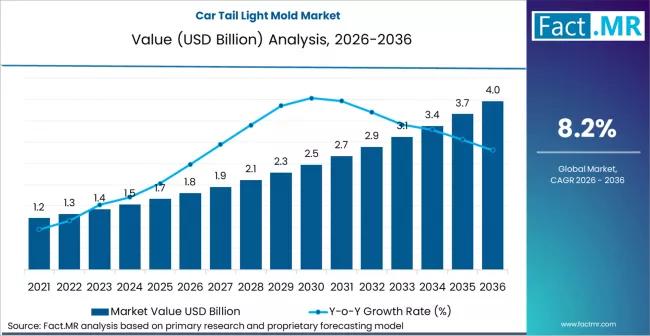
Car Tail Light Mold Market is Hoped-for USD 3.6 billion by 2036 | Fact.MR Report
The global car tail light mold market is navigating a high-design era, projected to grow from a valuation of USD 1.4 billion in 2026 to approximately USD 2.6 billion by 2036. This represents a compound annual growth rate (CAGR) of 6.4% over the forecast period.
The market is being fundamentally reshaped by the transition from simple bulbs to complex LED and OLED signatures, requiring high-precision multi-color and multi-material injection molding…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…