Press release
Global FDA Grade Weight-Loss Devices Market Analysis and Forecast, 2019-2028
The global FDA-grade weight-loss devices market is valued at a market value of US$ 6.8 billion in 2022 and is expected to grow at a significant CAGR of over 12% over the forecast period of 2019-2028.Market Scope and Report Overview
According to a deep-dive market assessment by RationalStat, the global FDA-grade weight-loss devices market has been analyzed on the basis of market segments, including type, application, and geography/regions (incl. North America, Latin America, Western Europe, Eastern Europe, Middle East & Africa, and Asia Pacific). The report also offers global and regional market sizing for the historical period of 2019-2022 and the forecast period of 2019-2028.
Market intelligence for the global FDA-grade weight-loss devices market covers market sizes on the basis of market value (US$/EUR Million) and volume ('000 units/tons/liters) by various products/services/ equipment, demand assessment across the key regions, customer sentiments, price points, cost structures, margin analysis across the value chain, financial assessments, historical and forecast data, key developments across the industry, import-export data, trade overview, components market by leading companies, etc.
In addition, the long-term sector and products/services 10-year outlook and its implications on the global FDA-grade weight-loss devices market. It also includes the industry's current state - Production Levels, Capacity Utilization, Tech quotient, etc. Key information will be manufacturing capacity by country, installed base, import volumes, market size, key players, market size, dynamics, market data, insights, etc.
Period Covered include data for 2019-2028 along with year-wise demand estimations
The FDA-grade weight-loss devices market report analyzes the market on the basis of global economic situations, regional geopolitics, import-export scenarios, trade duties, market developments, organic and inorganic strategies, mergers and acquisitions, product launches, government policies, new capacity addition, technological advancements, R&D investments, and new market entry, replacement rates, penetration rates, installed base/fleet size, global and regional production capacity, among others.
RationalStat offers market analysis and consulting studies on the basis of dedicated and robust desk/secondary research supported by a strong in-house data repository. In addition, the research leverages data based on the real-time insights gathered from primary interviews. Market estimations and insights are based on primary research (covering more than 240 entities) and secondary research by leveraging international benchmarking.
The global FDA-grade weight-loss devices market report also covers value chain and supply chain analysis that provides in-depth information about the value chain margins and the role of various stakeholders across the value chain. Market dynamics provided in the market study include market drivers, restraints/challenges, trends, and their impact on the market throughout the analysis period.
In the competition analysis section, the global FDA-grade weight-loss devices market provides a detailed competition benchmarking analysis based on the market share of the leading companies/brands/ producers/suppliers, a market structure overview with detailed company profiles of more than 25 players with their financials, product/service offerings, major developments, business models, etc. This enables, clients and report buyers to make strong, precise, and timely decisions.
Explore more about this report - Request for Sample and Scope of the Study
https://store.rationalstat.com/store/global-fda-grade-weight-loss-devices-market/#tab-ux_global_tab
Macroeconomic Scenario and the Impact of COVID-19 on Regional Economic Sentiment
In the latest RationalStat analysis, geopolitical conflicts and inflation are the cited economic risks, while concerns about the volatility across energy sectors prevail in Europe and other parts of the world. Some of the potential risks to the economic growth in the leading regions, including Asia Pacific, Europe, North America, the Middle East & Africa, and other developing regions, are inflation, volatile energy prices, supply chain disruptions, geopolitical instability, labor shortages, rising interest rates, and COVID-19 pandemic.
The global economy experienced heavy headwinds, throughout 2019-2021, as some countries witnessed subdued growth, while other countries continued to grapple with economic slowdowns. The COVID-19 pandemic has levied undue pressure across the majority of industries globally and has caused a major economic crisis in the US, India, Italy, the UK, Germany, India, Japan, South Korea, the UK, and many others. Besides, the exit of the UK from the European Union earlier in 2020 and the Russo-Ukraine war in 2022 exacerbated the ever-heightened global uncertainty.
In addition to this, the global economic growth slowed in 2022 to 3.3%, weaker than expected at the end of 2021, mainly weighed down by Russia's war in Ukraine and the associated cost-of-living crisis in many countries. However, improvement in economic activities during the forecast period is expected. Growth is projected to remain at lower rates in 2023 and 2024, at 2.6% and 2.9% respectively.
Competition Analysis and Market Structure
Some of the prominent players that contribute significantly to the global FDA-grade weight-loss devices market growth include Nokia Corporation, ReShape Medical, Helioscopie Medical Implants, Allurion, Spatz FGIA, Lexal, Obalon, Medsil, Endalis, Districlass Medical, and among others.
RationalStat has segmented the global FDA-grade weight-loss devices market based on type, application, and region
• Global FDA Grade Weight-Loss Devices Market Value (US$ Million), Volume ('000 units/tons), and Market Share (2019-2028) Analysis by Type
o Gastric Band
o Electrical Stimulation Systems
o Gastric Balloon Systems
o Gastric Emptying Systems
o Others
• Global FDA Grade Weight-Loss Devices Market Value (US$ Million), Volume ('000 units/tons), and Market Share (2019-2028) Analysis by Application
o Hospitals & Clinics
o Home Care
o Others
• Global FDA Grade Weight-Loss Devices Market Value (US$ Million), Volume ('000 units/tons), and Market Share (2019-2028) Analysis by Region
o North America FDA Grade Weight-Loss Devices Market
US
Canada
o Latin America FDA Grade Weight-Loss Devices Market
Brazil
Mexico
Rest of Latin America
o Western Europe FDA Grade Weight-Loss Devices Market
Germany
UK
France
Spain
Italy
Benelux
Nordic
Rest of Western Europe
o Eastern Europe FDA Grade Weight-Loss Devices Market
Russia
Poland
Hungary
Other CIS Countries
Rest of Eastern Europe
o Asia Pacific FDA Grade Weight-Loss Devices Market
China
Japan
India
South Korea
Australia
ASEAN
• Indonesia
• Thailand
• Philippines
• Vietnam
• Malaysia
• Rest of ASEAN
Rest of Asia Pacific
o Middle East & Africa FDA Grade Weight-Loss Devices Market
GCC
• Saudi Arabia (KSA)
• United Arab Emirates (UAE)
• Rest of the GCC
South Africa
Nigeria
Turkey
Rest of the Middle East & Africa
• Leading Companies and Market Players
o Nokia Corporation
o ReShape Medical
o Helioscopie Medical Implants
o Allurion
o Spatz FGIA
o Lexal
o Obalon
o Medsil
o Endalis
o Districlass Medical
For more information about this report https://store.rationalstat.com/store/global-fda-grade-weight-loss-devices-market/
Key Questions Answered in the FDA Grade Weight-Loss Devices Report:
• What will be the market value of the global FDA grade weight-loss devices market by 2028?
• What is the market size of the global FDA grade weight-loss devices market?
• What are the market drivers of the global FDA grade weight-loss devices market?
• What are the key trends in the global FDA grade weight-loss devices market?
• Which is the leading region in the global FDA grade weight-loss devices market?
• What are the major companies operating in the global FDA grade weight-loss devices market?
• What are the market shares by key segments in the global FDA grade weight-loss devices market?
Kimberly Shaw,
Content and Press Manager
RationalStat LLC
sales@rationalstat.com
Phone: +1 302 803 5429
RationalStat is an end-to-end global market intelligence and consulting company that provides comprehensive market research reports, customized strategy, and consulting studies. The company has sales offices in India, Mexico, and the US to support global and diversified businesses. The company has over 80 consultants and industry experts, developing more than 850 market research and industry reports for its report store annually.
RationalStat has strategic partnerships with leading data analytics and consumer research companies to cater to the client's needs. Additional services offered by the company include consumer research, country reports, risk reports, valuations and advisory, financial research, due diligence, procurement and supply chain research, data analytics, and analytical dashboards.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global FDA Grade Weight-Loss Devices Market Analysis and Forecast, 2019-2028 here
News-ID: 3066499 • Views: …
More Releases from RationalStat LLC
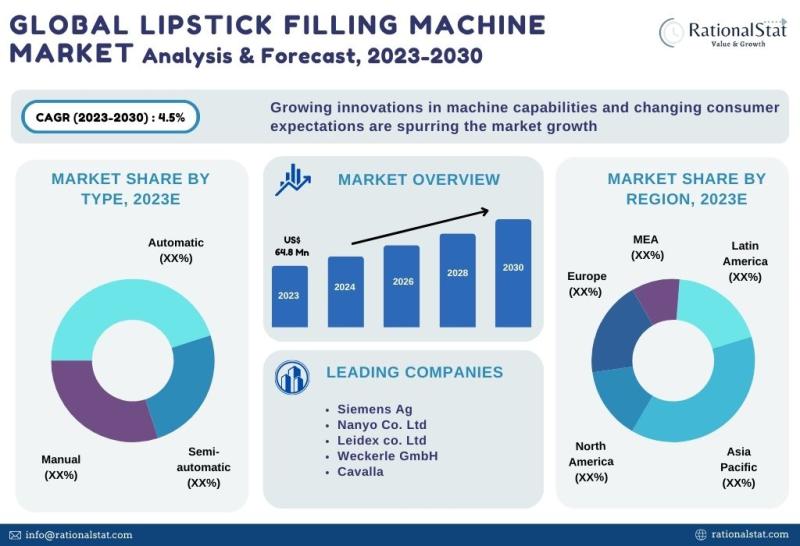
Latest Market Study | Global Lipstick Filling Machine Market Size, Share, & Fore …
The global lipstick filling machine market is expected to reach US$ 88.2 million by 2030, with an annual growth rate of more than 4.5%.
According to RationalStat's recent industry analysis, the Global Lipstick Filling Machine Market value is estimated at US$ 64.8 million in 2023 and is expected to rise at a strong CAGR of over 4.5% over the forecast period of 2023-2030.
Market Definition, Market Scope, and Report Overview
A lipstick…
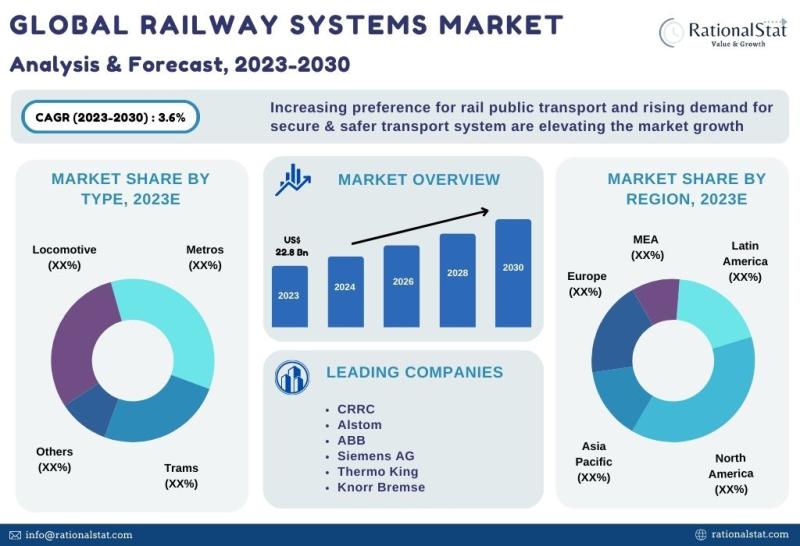
Published Market Report | Global Railway Systems Market Size, Share, & Forecast …
The global railway systems market is expected to reach US$ 29.2 billion by 2030, with an annual growth rate of more than 3.6%.
According to RationalStat's recent industry analysis, the Global Railway Systems Market value is estimated at US$ 22.8 billion in 2023 and is expected to rise at a strong CAGR of over 3.6% over the forecast period of 2023-2030.
Market Definition, Market Scope, and Report Overview
Railway systems, also known…
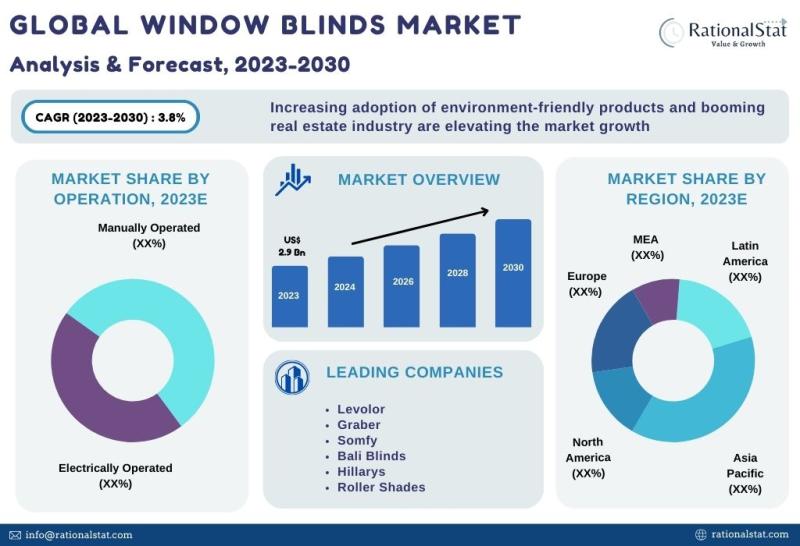
Published Market Report | Global Window Blinds Market Size, Share, & Forecast 20 …
The global window blinds market is expected to reach US$ 3.7 billion by 2030, with an annual growth rate of more than 3.8%.
According to RationalStat's most recent industry analysis, the Global Window Blinds Market value is estimated at US$ 2.9 billion in 2023 and is expected to rise at a strong CAGR of over 3.8% over the forecast period of 2023-2030.
Market Definition, Market Scope, and Report Overview
Window blinds are…
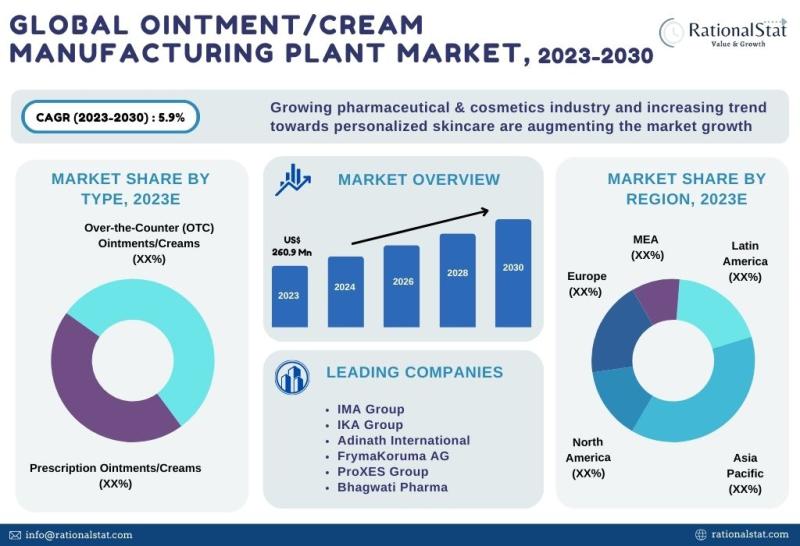
Ointment and Cream Manufacturing Plant Market Report 2023 | Ointment and Cream M …
The global ointment and cream manufacturing plant market is expected to approach US$ 388.7 million by 2030, with an annual growth rate of more than 5.9%
Global Ointment and Cream Manufacturing Plant Market is valued at US$ 260.9 million in 2023 and is expected to grow at a significant CAGR of over 5.9% over the forecast period of 2023-2030, according to the published market report by RationalStat
Market Definition, Market…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
