Press release
TIGIT Inhibitors Clinical Trials, Drug development, Latest FDA Approvals and Treatment Options by Delveinsight
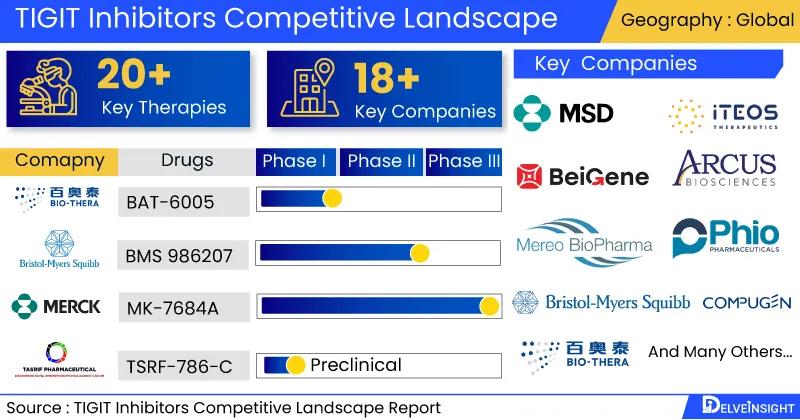
TIGIT Inhibitors pipeline constitutes 18+ key companies continuously working towardsdeveloping 20+ TIGIT Inhibitors treatment therapies, analyzes DelveInsight
T cell immunoglobulin and ITIM domain (TIGIT) is an inhibitory receptor expressed on lymphocytes that was recently propelled under the spotlight as a major emerging target in cancer immunotherapy. TIGIT interacts with CD155 expressed on antigen‐presenting cells or tumor cells to down‐regulate T cell and natural killer (NK) cell functions. TIGIT has emerged as a key inhibitor of anti‐tumor responses that can hinder multiple steps of the cancer immunity cycle.
DelveInsights, "TIGIT Inhibitors Competitive landscape, 2022," report provides
comprehensive insights about 18+ companies and TIGIT Inhibitors drugs in TIGIT
inhibitors Competitive landscape. It covers the therapeutics assessment by product type,
stage, route of administration, and molecule type. It further highlights the inactive pipeline
products in this space.
To know more about the TIGIT Inhibitors Competitive landscape report, click here:
https://www.delveinsight.com/report-store/tigit-inhibitors-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key Takeaways from the TIGIT Inhibitors Competitive landscape report:
• Leading TIGIT Inhibitors Companies working in the market are Merck Sharp & Dohme, Bristol-Myers Squibb, BeiGene, Arcus Biosciences, iTeos Therapeutics, Mereo BioPharma, Phio Pharmaceuticals, Bio-Thera Solutions, Compugen.
• Key TIGIT Inhibitors therapies in various stages of development include Ociperlimab+ Tislelizumab, MK-7684A, Domvanalimab+ Zimberelimab, BMS 986207, BAT-6005, AGEN 1777+ PD-1 Inhibitor, PH 804, TSRF-786-C and Others.
• In December 2021, Bio-Thera Solutions a commercial-stage pharmaceutical company announced that dosing has begun in Phase I clinical study to compare the pharmacokinetics and safety of BAT6005, a monoclonal antibody targeting TIGIT in cancer patient volunteers.
DelveInsight's TIGIT Inhibitors Report covers around 200+ products under different phases
of clinical development like
• Assessment by TIGIT Inhibitors Product Type
• Assessment by Stage and Product Type of TIGIT Inhibitors
• Assessment by Route of Administration
• Assessment by Stage and Route of Administration of TIGIT Inhibitors
• Assessment by TIGIT Inhibitors Molecule Type
• Assessment by Stage and Molecule Type of TIGIT Inhibitors
Emerging TIGIT Inhibitors Drugs Under Different Phases of Clinical Development Include:
Some of the TIGIT Inhibitors therapies are Ociperlimab, BeiGene and Many Others.
Further TIGIT Inhibitors product details are provided in the report. Download the
TIGIT Inhibitors Pipeline report to learn more about the emerging TIGIT Inhibitors therapies at: https://www.delveinsight.com/report-store/tigit-inhibitors-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key companies in the TIGIT Inhibitors Therapeutics Market:
Some of the TIGIT Inhibitors Companies working in the market are Merck Sharp & Dohme, Bristol-Myers Squibb, BeiGene, Arcus Biosciences, iTeos Therapeutics, Mereo BioPharma, Phio Pharmaceuticals, Bio-Thera Solutions, Compugen.
Request for Sample PDF Report to know in detail about the recent developments and
advancements in TIGIT Inhibitors clinical trials - https://www.delveinsight.com/sample-request/tigit-inhibitors-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Table of Content (TOC)
1. TIGIT Inhibitors Pipeline Report Introduction
2. TIGIT Inhibitors Pipeline Report Executive Summary
3. TIGIT Inhibitors Pipeline: Overview
4. Analytical Perspective In-depth Commercial Assessment
5. TIGIT Inhibitors Clinical Trial Therapeutics
6. TIGIT Inhibitors Pipeline: Late Stage Products (Pre-registration)
7. TIGIT Inhibitors Pipeline: Late Stage Products (Phase III)
8. TIGIT Inhibitors Pipeline: Mid Stage Products (Phase II)
9. TIGIT Inhibitors Pipeline: Early Stage Products (Phase I)
10. TIGIT Inhibitors Pipeline Therapeutic Assessment
11. Inactive Products in the TIGIT Inhibitors Pipeline
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Key TIGIT Inhibitors Companies
14. Key Products in the TIGIT Inhibitors Pipeline
15. Unmet Needs
16. TIGIT Inhibitors Market Drivers and Barriers
17. Future Perspectives and Conclusion
18. Analyst Views
19. Appendix
Download Sample PDF Report to know more about @ https://www.delveinsight.com/report-store/tigit-inhibitors-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Trending Reports:
HPV-Induced Cancers Market:
https://www.delveinsight.com/report-store/hpv-induced-cancers-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Ventricular Fibrillation Market:
https://www.delveinsight.com/report-store/ventricular-fibrillation-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Diagnostic Imaging Equipment Market:
https://www.delveinsight.com/report-store/diagnostic-imaging-equipment-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Aortic Aneurysm Stent Grafts Market:
https://www.delveinsight.com/report-store/aortic-aneurysm-stent-grafts-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Atherectomy Devices Market:
https://www.delveinsight.com/report-store/atherectomy-devices-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email: info@delveinsight.com
Phone: 9193216187
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/consulting
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research Firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release TIGIT Inhibitors Clinical Trials, Drug development, Latest FDA Approvals and Treatment Options by Delveinsight here
News-ID: 2807358 • Views: …
More Releases from DelveInsight Business Research

Gastric Cancer Market to Exceed USD 700 Million in HER2+ Segment by 2034 Across …
Gastric cancer is the fifth most common malignancy worldwide, with incidence varying by region and highest rates reported in Eastern Asia and Eastern Europe. Japan records the highest burden among the 7MM, with about 129,500 cases in 2024. Many patients, particularly in Western countries, are diagnosed at advanced (Stage IV) stages, while Japan detects more cases early (Stage I). In the US, around 35% present with metastatic disease at diagnosis,…

Myelofibrosis Market to Double by 2034, Surpassing USD 5.6 Billion Across 7MM wi …
The Myelofibrosis market across the seven major markets (7MM) is anticipated to experience significant growth, rising from approximately USD 2,602 million in 2025 to nearly USD 5,638 million by 2034, reflecting a compound annual growth rate (CAGR) of about 9% during the forecast period.
In 2024, the market was valued at around USD 2.2 billion, with the United States leading at nearly USD 1.7 billion, far surpassing the EU4, the United…

Triple-Negative Breast Cancer Clinical Trial Landscape Expands With 170+ Pipelin …
An expanding portfolio of novel therapies for triple-negative breast cancer (TNBC) including TECENTRIQ (Hoffmann-La Roche), IPI-549 (Infinity Pharmaceuticals), Leronlimab (CytoDyn), MDNA11 (Medicenna Therapeutics), VIP236 (Vincerx Pharma), and CFI-400945 (Treadwell Therapeutics) is anticipated to significantly influence market expansion and transform the TNBC treatment landscape.
DelveInsight's Triple Negative Breast Cancer Pipeline Insight report offers an extensive evaluation of more than 165 companies and over 170 therapeutic candidates advancing through the TNBC pipeline. The…

Asthma Market to Witness Steady Growth Through 2034 Driven by Novel Biologics an …
DelveInsight's latest Asthma Market Insights, Epidemiology, and Market Forecast - 2034 report provides an in-depth evaluation of current treatment practices, emerging asthma drugs, market share of individual therapies, and forecasted market trends across the seven major markets (7MM) - the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
The asthma market is expected to experience sustained growth over the next decade, supported by the launch of…
More Releases for TIGIT
Development and Optimization of Next-Generation TIGIT Antibodies
The development and optimization of next-generation TIGIT antibodies represent a significant advancement in cancer immunotherapy. These antibodies are designed to target the TIGIT immune checkpoint more effectively, offering improved therapeutic potential for enhancing anti-tumor immune responses.
Download Report
https://www.kuickresearch.com/report-anti-tigit-antibody-anti-tigit-antibodies-fda-approved-tigit-antibody-tigit-inhibitors-tigit-drugs-approved-tigit-expression-tigit-ligand-tigit-gene
The initial development of TIGIT antibodies focused on blocking the interaction between TIGIT and its ligands, CD155 and CD112. By preventing this binding, the inhibitory signals transmitted by TIGIT are lifted, allowing immune cells…
The Future of TIGIT Antibodies in Personalized Cancer Treatment
The future of TIGIT antibodies in personalized cancer treatment looks promising, with the potential to revolutionize the way we approach cancer therapy. Personalized medicine aims to tailor treatments based on individual patient characteristics, including genetic, molecular, and immune profiles. TIGIT antibodies, by targeting a specific immune checkpoint, offer a new avenue for customizing cancer treatment and improving patient outcomes.
Download Report
https://www.kuickresearch.com/report-anti-tigit-antibody-anti-tigit-antibodies-fda-approved-tigit-antibody-tigit-inhibitors-tigit-drugs-approved-tigit-expression-tigit-ligand-tigit-gene
TIGIT, an inhibitory receptor expressed on T cells, NK cells, and…
Comparative Effectiveness of TIGIT Antibodies and Other Checkpoint Inhibitors
The comparative effectiveness of TIGIT antibodies and other checkpoint inhibitors is a topic of significant interest in the field of cancer immunotherapy. Immune checkpoint inhibitors have revolutionized cancer treatment by unleashing the immune system's ability to attack tumors. While PD-1 and CTLA-4 inhibitors have shown remarkable success, TIGIT antibodies offer a new approach with potentially complementary mechanisms.
Download Report
https://www.kuickresearch.com/report-anti-tigit-antibody-anti-tigit-antibodies-fda-approved-tigit-antibody-tigit-inhibitors-tigit-drugs-approved-tigit-expression-tigit-ligand-tigit-gene
PD-1 inhibitors, such as pembrolizumab and nivolumab, block the PD-1 receptor on T…
Biomarkers for Predicting Response to TIGIT Antibody Therapy
Identifying biomarkers for predicting response to TIGIT antibody therapy is crucial for optimizing treatment outcomes and personalizing cancer therapy. Biomarkers can help select patients who are most likely to benefit from TIGIT blockade, thereby improving the efficacy and reducing unnecessary exposure to the therapy.
Download Report
https://www.kuickresearch.com/report-anti-tigit-antibody-anti-tigit-antibodies-fda-approved-tigit-antibody-tigit-inhibitors-tigit-drugs-approved-tigit-expression-tigit-ligand-tigit-gene
One of the most promising biomarkers for TIGIT antibody therapy is the expression level of CD155, the primary ligand for TIGIT. High levels of CD155 expression…
Innovations in Immunotherapy The Rise of Anti TIGIT Antibodies
Innovations in immunotherapy have transformed the landscape of cancer treatment, offering new hope to patients who previously had limited options. Among these innovations, the rise of anti-TIGIT antibodies represents a significant advancement in the field, providing a novel approach to enhancing the immune system's ability to combat cancer. Anti-TIGIT antibodies are a new class of immune checkpoint inhibitors that target TIGIT, an inhibitory receptor expressed on T-cells, natural killer (NK)…
Anti TIGIT Antibodies Revolutionizing Immunotherapy
Anti-TIGIT antibodies are at the forefront of a revolution in immunotherapy, representing a new class of immune checkpoint inhibitors that have the potential to transform the treatment landscape for cancer. Immunotherapy, which harnesses the body's immune system to fight cancer, has already made significant strides with the introduction of PD-1, PD-L1, and CTLA-4 inhibitors. However, the discovery and development of anti-TIGIT antibodies add a new dimension to this field, offering…