Press release
Spasticity Clinical Pipeline 2025: A Comprehensive Review of 12+ Emerging Therapies from 10+ key Players, says DelveInsight
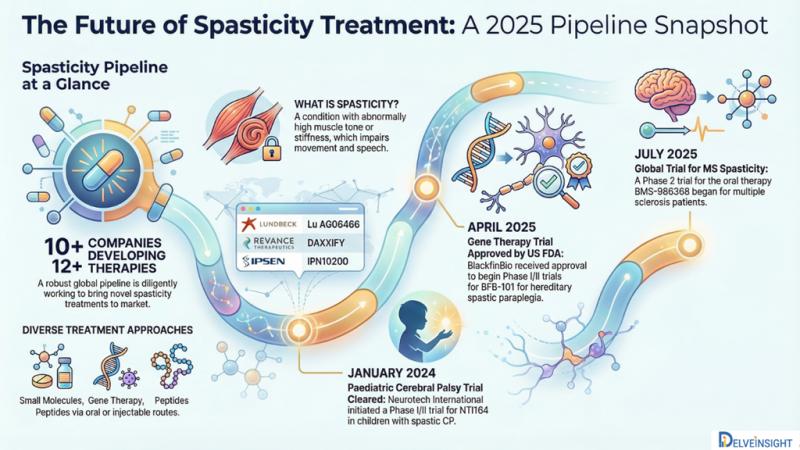
According to DelveInsight's latest analysis, the global Spasticity pipeline features more than 10 active pharmaceutical companies that are advancing over 12 investigational therapies. The report offers a detailed evaluation of clinical trials, mechanisms of action, therapeutic approaches, routes of administration, and major developmental activities shaping the Spasticity treatment landscape.DelveInsight's report, "Spasticity Pipeline Insight, 2025," delivers an extensive overview of the current clinical development environment and the future outlook for the Spasticity market.
The study provides an in-depth commercial and clinical review of pipeline candidates from preclinical research to marketed products. It includes comprehensive drug profiles covering mechanisms of action, clinical trial updates, regulatory milestones such as NDAs, and product development activities, including technological advancements, collaborations, licensing agreements, mergers and acquisitions, funding details, designations, and other key updates.
Download free sample report to discover emerging therapies @ https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=openpr&utm_medium=promotion&utm_campaign=apr
Key Highlights from the Spasticity Pipeline Report
• Global pharmaceutical companies are actively engaged in developing innovative Spasticity therapies, achieving notable progress in recent years.
• Leading players developing treatments for Spasticity include Lundbeck, Revance Therapeutics, Ipsen, Saol Therapeutics, Huons, Supernus Pharma, Sun Pharma, Acorda Therapeutics, Merz Pharmaceuticals GmbH, Medy-Tox, XenoPort, Inc., Medtronic Neuro, Daewoong Pharma, and several others.
• Prominent emerging therapies in clinical development include Lu AG06466, DAXXIFY, IPN10200, SL-1002, HU-014, MYOBLOC, SPARC1103, Tizanidine, CORETOX®, XP19986 SR1, intrathecal baclofen, Botulinum toxin type A, among others each expected to influence the Spasticity treatment landscape significantly in the coming years.
Recent Spasticity Clinical and Regulatory Developments
• July 2025: A global Phase 2 trial, BALANCE-MSS-1 (NCT06782490), began enrolling approximately 200 adults with multiple sclerosis-related spasticity to evaluate Bristol Myers Squibb's oral candidate BMS-986368. The study spans the U.S., Europe, Australia, Canada, and Puerto Rico and is sponsored by Celgene, now operating under BMS.
•april 2025: The U.S. FDA cleared BlackfinBio's IND application for a Phase I/II study of BFB-101, an AAV gene therapy targeting hereditary spastic paraplegia type 47 (SPG47). The candidate holds orphan drug and rare pediatric disease designations, addressing AP4B1 gene mutations leading to progressive spasticity and developmental impairments in children.
• March 2025: BlackfinBio announced the submission of its IND application for the same AAV gene therapy designed for treating SPG47.
• January 2024: Neurotech International received approval from the Human Research Ethics Committee (HREC) and Australia's Therapeutic Goods Administration (TGA) to begin a Phase I/II trial of NTI164 in children with spastic cerebral palsy the most prevalent form of CP.
Get a Free Sample PDF Report to know more about Spasticity Pipeline Therapeutic Assessment-https://www.delveinsight.com/report-store/spasticity-pipeline-insight?utm_source=openpr&utm_medium=promotion&utm_campaign=apr
Overview of Spasticity
Spasticity is characterized by abnormal increases in muscle tone or stiffness, leading to impaired mobility, speech challenges, and gait abnormalities. It typically results from neurological damage or disruption within the brain or spinal cord that affects the pathways regulating muscle movement.
Emerging Spasticity Therapies in the Pipeline
Key candidates under development include:
• Lu AG06466 - Lundbeck
• DAXXIFY - Revance Therapeutics
• IPN10200 - Ipsen
• HU-014 - Huons
• SL-1002 - Saol Therapeutics
• MYOBLOC - Supernus Pharma
• SPARC1103 - Sun Pharma
• Tizanidine - Acorda Therapeutics
• Botulinum toxin type A - Merz Pharmaceuticals GmbH
• CORETOX® - Medy-Tox
• XP19986 SR1 - XenoPort, Inc.
• Intrathecal baclofen - Medtronic Neuro
Further Spasticity product details are provided in the report. Download the Spasticity pipeline report to learn more about the emerging Spasticity therapies - https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=openpr&utm_medium=promotion&utm_campaign=apr
Spasticity Drugs Routes of Administration (ROA)
The Spasticity pipeline includes therapies delivered through:
• Oral
• Parenteral
• Intravenous
• Subcutaneous
• Topical
Spasticity Drugs by Molecule Types
Products in development span a diverse range of molecule classes:
• Monoclonal antibodies
• Peptides
• Polymers
• Small molecules
• Gene therapies
Spasticity Therapeutic Assessments Covered
• Analysis by product type
• Stage-wise product segmentation
• Route of administration breakdown
• Stage-wise ROA evaluation
• Molecule type segmentation
• Stage-wise molecule classification
DelveInsight's report profiles 12+ candidates across all phases, including:
• Phase III (late-stage)
• Phase II (mid-stage)
• Phase I (early-stage)
• Preclinical and discovery assets
• Discontinued and inactive programs
Download Sample PDF Report to know more about Spasticity drugs and therapies - https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=openpr&utm_medium=promotion&utm_campaign=apr
Spasticity Pipeline Analysis
The report explores:
• Company-level therapeutic portfolios, including active and discontinued assets
• Stage-based analysis of therapeutic candidates
• Therapies categorized by route of administration, mechanism of action, monotherapy/combination status, and molecular structure
• Extensive details on partnerships, academia-industry collaborations, licensing agreements, and funding
• Data sourced from proprietary databases, clinical trial registries, corporate websites, SEC submissions, investor materials, conference proceedings, and validated third-party sources
Spasticity Market Drivers
• Rising incidence of stroke, MS, and brain injuries
• Growing R&D focus to identify effective and targeted Spasticity treatments
Spasticity Market Barriers
• High treatment costs
• Limited disease awareness in developing markets
• Additional challenges affecting therapy adoption
Scope of the Spasticity Market Report
• Coverage: Global
• Leading Companies: Lundbeck, Revance Therapeutics, Ipsen, Saol Therapeutics, Huons, Supernus Pharma, Sun Pharma, Acorda Therapeutics, Merz Pharmaceuticals GmbH, Medy-Tox, XenoPort, Medtronic Neuro, Daewoong Pharma, and others
• Key Therapies: Lu AG06466, DAXXIFY, IPN10200, SL-1002, HU-014, MYOBLOC, SPARC1103, Tizanidine, CORETOX®, XP19986 SR1, intrathecal baclofen, Botulinum toxin type A, etc.
• Therapeutic Coverage: Current and emerging therapies
• Market Dynamics: Detailed evaluation of drivers and barriers influencing the Spasticity market
Request for Sample PDF Report for Spasticity Pipeline Assessment and clinical trials - https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=openpr&utm_medium=promotion&utm_campaign=apr
Contact Us:
Ankit Nigam
Manager Marketing
info@delveinsight.com
+14699457679
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Spasticity Clinical Pipeline 2025: A Comprehensive Review of 12+ Emerging Therapies from 10+ key Players, says DelveInsight here
News-ID: 4312387 • Views: …
More Releases from DelveInsight Business Research

Epidermolysis Bullosa Market: Strong Pharma Growth Forecast Through 2034 - Delve …
The Epidermolysis Bullosa market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Epidermolysis Bullosa pipeline products will significantly revolutionize the Epidermolysis Bullosa market dynamics.
DelveInsight's "Epidermolysis Bullosa Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Epidermolysis Bullosa, historical and forecasted epidemiology as well as the Epidermolysis Bullosa market trends in the United…

Anal Cancer Market: Accelerating Growth and Pipeline Impact by 2034 - DelveInsig …
DelveInsight's "Anal Cancer Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Anal Cancer, historical and forecasted epidemiology as well as the Anal Cancer market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
The latest healthcare forecast report provides an in-depth analysis of Anal Cancer, offering comprehensive insights into the Anal Cancer revenue trends, prevalence, and treatment landscape. The…

Short Bowel Syndrome Market: High-Growth Opportunities for Investors to 2034 - D …
DelveInsight's "Short Bowel Syndrome Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Short Bowel Syndrome, historical and forecasted epidemiology as well as the Short Bowel Syndrome market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Short Bowel Syndrome market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Short Bowel Syndrome…

Gastroparesis Market: Rapid Increment Driven by Innovation by 2034 - DelveInsigh …
DelveInsight's "Gastroparesis Market Insights, Epidemiology, and Market Forecast-2034′′ report offers an in-depth understanding of the Gastroparesis, historical and forecasted epidemiology as well as the Gastroparesis market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.
To Know in detail about the Gastroparesis market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Gastroparesis Market Forecast
https://www.delveinsight.com/sample-request/gastroparesis-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Some of the key facts of the Gastroparesis…
More Releases for Spasticity
Spasticity Market: Rapid Growth & Investment Outlook to 2032 - DelveInsight
The Spasticity market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Spasticity pipeline products will significantly revolutionize the Spasticity market dynamics.
DelveInsight's "Spasticity Market Insights, Epidemiology, and Market Forecast-2032′′ report offers an in-depth understanding of the Spasticity, historical and forecasted epidemiology as well as the Spasticity market trends in the United States, EU5 (Germany, Spain, Italy, France,…
Spasticity Market 2025-2034 Business Outlook, Critical Insight and Growth
Introduction
Spasticity is a motor disorder characterized by involuntary muscle stiffness, spasms, and exaggerated reflexes caused by damage to the central nervous system (CNS). It frequently occurs as a complication of stroke, multiple sclerosis (MS), cerebral palsy, traumatic brain or spinal cord injury, and other neurological conditions. Spasticity can severely impact mobility, daily functioning, and quality of life, making it a significant healthcare burden worldwide.
Treatment approaches combine pharmacological agents, intrathecal therapies,…
Muscle Spasticity Market Emerging Trends and Growth Prospects 2034
Introduction
Muscle spasticity is a condition characterized by involuntary muscle stiffness, spasms, and increased tone, often resulting from neurological disorders such as multiple sclerosis, cerebral palsy, stroke, or traumatic brain and spinal cord injuries. It significantly impacts mobility and quality of life, making effective management essential. With the rising incidence of neurological diseases and advancements in physiotherapy, drug therapies, and medical devices, the global muscle spasticity market is witnessing steady expansion.
According…
Spasticity Drugs Market Segments, Growth and Trends by Forecast by 2031
The report is segmented By Drug Type (Baclofen, Botulinum Toxin, Diazepam, Tizanidine, Dantrolene Sodium, and Others), Route of Administration (Oral, Intramuscular, and Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Get Sample Copy -https://www.theinsightpartners.com/sample/TIPRE00040811/?utm_source=OpenPR&utm_medium=10831
Major Companies operating in the Spasticity Drugs Market are:
Ipsen Pharma
Allergan
Acorda Therapeutics,…
Spasticity Drugs Top Companies Regional and Data Analysis to 2031
The Spasticity Drugs Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits.
Download PDF Brochure at: https://www.theinsightpartners.com/sample/TIPRE00040811/?utm_source=OpenPR&utm_medium=10129
Major Companies operating in the Spasticity Drugs Market are:
• Ipsen Pharma
• Allergan
• Acorda Therapeutics, Inc.
• Merz Pharma
• Teva Pharmaceutical Industries Ltd.
• Novartis AG
• Sun Pharmaceutical Industries Ltd.
• Beximco Pharmaceuticals Ltd.
• Johnson & Johnson Private Limited
• Zydus Cadila
Contact Us:
If you have any queries about this report or if…
Spasticity Treatment Market Expecting Huge Demand in Upcoming Years
The Global Spasticity Treatment Market size is estimated at $5.7 Billion in 2025 and is forecast to register an annual growth rate (CAGR) of 4.9% to reach $8.8 Billion by 2034. Download now
The latest study released on the Global Spasticity Treatment Market by USD Analytics Market evaluates market size, trend, and forecast to 2034. The Spasticity Treatment market study covers significant research data and proofs to be a handy resource…