Press release
External Remote Patient Monitoring Devices Market Report- Growth, Share and Analysis of Key Players: ATLASense Biomed Ltd, Barron Associates
The External Remote Patient Monitoring Devices Market research study also examines the global and regional breakdown of the industry, its features, market shares, policies, and patterns, and the constantly changing global market environment. External Remote Patient Monitoring Devices Market research summary also incorporates the overview of the primary industry’s trend and the world market’s estimated value and volume depending upon regional evaluation. Moreover, the business offerings mentioned in External Remote Patient Monitoring Devices Market report represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.Get FREE PDF Sample of the Report @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4998951
External Remote Patient Monitoring Devices Market Report provides comprehensive information about the External Remote Patient Monitoring Devices pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
External remote patient monitoring devices retrieves the data from the external measurement device (like glucose meters, blood pressure monitors, pulse oximeters, weight scales, ECG) and transmit it, through a wireless system to a monitoring station or a physicians office for further analysis and interpretation.
Scope of this Report-
- Extensive coverage of the External Remote Patient Monitoring Devices under development
- External Remote Patient Monitoring Devices Market Report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
- External Remote Patient Monitoring Devices Market Report reviews the major players involved in the development of External Remote Patient Monitoring Devices and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- External Remote Patient Monitoring Devices Market Report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry
Reasons to Buy this Report-
External Remote Patient Monitoring Devices Market Report enables you to -
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of External Remote Patient Monitoring Devices under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the products current stage of development, territory and estimated launch date
Get Discount on Purchase this Report @ https://www.reportsnreports.com/purchase.aspx?name=4998951
List of Tables
External Remote Patient Monitoring Devices - Pipeline Products by Stage of Development
External Remote Patient Monitoring Devices - Pipeline Products by Territory
External Remote Patient Monitoring Devices - Pipeline Products by Regulatory Path
External Remote Patient Monitoring Devices - Pipeline Products by Estimated Approval Date
External Remote Patient Monitoring Devices - Ongoing Clinical Trials
External Remote Patient Monitoring Devices Companies - Pipeline Products by Stage of Development
External Remote Patient Monitoring Devices - Pipeline Products by Stage of Development
Active4D Inc Pipeline Products & Ongoing Clinical Trials Overview
Wearable Sensor Device - Product Status
Wearable Sensor Device - Product Description
ActiveCare Inc Pipeline Products & Ongoing Clinical Trials Overview
ActiveOne+ - Product Status
ActiveOne+ - Product Description
Agali Technologies, Inc. Pipeline Products & Ongoing Clinical Trials Overview
VigilCare - Product Status
VigilCare - Product Description
AirStrip Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview
Airstrip mHealth Remote Care Information System - Product Status
Airstrip mHealth Remote Care Information System - Product Description
ALR Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview
Health-e-Connect System - Blood Pressure Meter - Product Status
Health-e-Connect System - Blood Pressure Meter - Product Description
Health-e-Connect System - Body Composition Monitor - Product Status
Health-e-Connect System - Body Composition Monitor - Product Description
Health-e-Connect System - Electrocardiogram - Product Status
Health-e-Connect System - Electrocardiogram - Product Description
Health-e-Connect System - Peak Flow Meter - Product Status
Health-e-Connect System - Peak Flow Meter - Product Description
Health-e-Connect System - Pulse Oximeter - Product Status
Health-e-Connect System - Pulse Oximeter - Product Description
Health-e-Connect System - Respiratory Health Management - Product Status
Health-e-Connect System - Respiratory Health Management - Product Description
Apogee Technology Inc Pipeline Products & Ongoing Clinical Trials Overview
IntellaPAL - Product Status
IntellaPAL - Product Description
Apple Inc Pipeline Products & Ongoing Clinical Trials Overview
Apple Watch - Glucose Monitoring - Product Status
Apple Watch - Glucose Monitoring - Product Description
ARC Devices Ltd. Pipeline Products & Ongoing Clinical Trials Overview
MedTemp RPM - Product Status
MedTemp RPM - Product Description
Aseptika Ltd Pipeline Products & Ongoing Clinical Trials Overview
BuddyWOTCH - Product Status
BuddyWOTCH - Product Description
Aster Labs Inc Pipeline Products & Ongoing Clinical Trials Overview
Activlink Insole - Product Status
Activlink Insole - Product Description
Aster Labs Inc - Ongoing Clinical Trials Overview
Activlink Insole - Accurate WiFi-based Localization of Dementia Patients for Caregiver Support: Phase II
ATLASense Biomed Ltd Pipeline Products & Ongoing Clinical Trials Overview
PolyMonitor - Product Status
PolyMonitor - Product Description
Barron Associates, Inc. Pipeline Products & Ongoing Clinical Trials Overview
FallCall System - Product Status
FallCall System - Product Description
TELEHOME System - Product Status
TELEHOME System - Product Description
BioSensics LLC Pipeline Products & Ongoing Clinical Trials Overview
HDWear - Product Status
HDWear - Product Description
Wrist-Worn Sensor - Product Status
Wrist-Worn Sensor - Product Description
BioSensics LLC - Ongoing Clinical Trials Overview
Wrist-Worn Sensor - Wrist-worn Sensors for Tele-rehabilitation of the Hemiparetic Upper Extremity: A Feasibility Study
Biotricity Inc Pipeline Products & Ongoing Clinical Trials Overview
Sleep Apnea Device - Product Status
Sleep Apnea Device - Product Description
Brain Tunnelgenix Technologies Corp Pipeline Products & Ongoing Clinical Trials Overview
BTT Wireless Device - Product Status
BTT Wireless Device - Product Description
BrightOutcome Inc. Pipeline Products & Ongoing Clinical Trials Overview
SymptomCareAnywhere (SCA) System - Product Status
SymptomCareAnywhere (SCA) System - Product Description
CardieX Ltd Pipeline Products & Ongoing Clinical Trials Overview
PPG Sensor - Product Status
PPG Sensor - Product Description
Chronolife Pipeline Products & Ongoing Clinical Trials Overview
KeeSense - Product Status
KeeSense - Product Description
KeeSense - Joint Diseases - Product Status
KeeSense - Joint Diseases - Product Description
Cnoga Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview
Smart Wearable Capsule - Product Status
Smart Wearable Capsule - Product Description
Consensus Orthopedics Inc Pipeline Products & Ongoing Clinical Trials Overview
TracPatch ACL - Product Status
TracPatch ACL - Product Description
TracPatch Hip - Product Status
TracPatch Hip - Product Description
TracPatch Shoulder - Product Status
TracPatch Shoulder - Product Description
TracPatch Spine - Product Status
TracPatch Spine - Product Description
CorTronix Biomedical Advancement Technologies Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
CorTab Medical Telemetry System - Product Status
CorTab Medical Telemetry System - Product Description
Curasis LLC Pipeline Products & Ongoing Clinical Trials Overview
Tele-EaT Sensor - Product Status
Tele-EaT Sensor - Product Description
Curasis LLC - Ongoing Clinical Trials Overview
Tele-EaT Sensor - Development and Validation of Mechanically Compliant Wearable Monitoring Systems for Swallowing Function and Disorders
Deep Breeze Ltd Pipeline Products & Ongoing Clinical Trials Overview
Breeze@home - Product Status
Breeze@home - Product Description
Eccrine Systems Inc Pipeline Products & Ongoing Clinical Trials Overview
Non-Invasive Sweat Sensor - Product Status
Non-Invasive Sweat Sensor - Product Description
Non-Invasive Wearable Device - Cognitive Status - Product Status
Non-Invasive Wearable Device - Cognitive Status - Product Description
Non-Invasive Wearable Device - Depression - Product Status
Non-Invasive Wearable Device - Depression - Product Description
Non-Invasive Wearable Device - Infection - Product Status
Non-Invasive Wearable Device - Infection - Product Description
Epicore Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview
Sweat Sensor - Product Status
Sweat Sensor - Product Description
Freescale Semiconductor, Ltd. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
Medical Kiosk - Product Status
Medical Kiosk - Product Description
Grey Innovation Pty Ltd Pipeline Products & Ongoing Clinical Trials Overview
Osmetric - Product Status
Osmetric - Product Description
Guide Analytics Inc. Pipeline Products & Ongoing Clinical Trials Overview
Ankle Bracelet Monitor - Product Status
Ankle Bracelet Monitor - Product Description
Hydrostasis Inc Pipeline Products & Ongoing Clinical Trials Overview
Wearable Hydration Monitoring System - Product Status
Wearable Hydration Monitoring System - Product Description
iBeat Inc Pipeline Products & Ongoing Clinical Trials Overview
iBeat Heart Watch - Product Status
iBeat Heart Watch - Product Description
Ibridge Medical, LLC Pipeline Products & Ongoing Clinical Trials Overview
iBridge Device - Product Status
iBridge Device - Product Description
Inspire Living, Inc. Pipeline Products & Ongoing Clinical Trials Overview
Inspire Monitor - Product Status
Inspire Monitor - Product Description
Itamar Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview
Wrist-Worn Device - Sleep Apnea - Product Status
Wrist-Worn Device - Sleep Apnea - Product Description
JointMetrix Medical, LLC Pipeline Products & Ongoing Clinical Trials Overview
Joint Monitoring System - Product Status
Joint Monitoring System - Product Description
Kimberly-Clark Corp Pipeline Products & Ongoing Clinical Trials Overview
Heat Stress Sensor - Product Status
Heat Stress Sensor - Product Description
KYOCERA Medical Corp (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
Wireless Headset - Product Status
Wireless Headset - Product Description
Lifelong Technologies, LLC Pipeline Products & Ongoing Clinical Trials Overview
Step Activity Monitor - Product Status
Step Activity Monitor - Product Description
MC10 Inc Pipeline Products & Ongoing Clinical Trials Overview
NIMBLE Patch - Parkinson's Disease - Product Status
NIMBLE Patch - Parkinson's Disease - Product Description
Michigan State University Pipeline Products & Ongoing Clinical Trials Overview
Wearable Sensor - Product Status
Wearable Sensor - Product Description
Montana State University Pipeline Products & Ongoing Clinical Trials Overview
Non-Contact Physiological Monitoring System - Product Status
Non-Contact Physiological Monitoring System - Product Description
National University of Singapore Pipeline Products & Ongoing Clinical Trials Overview
Wireless Vital Signs Patch - Product Status
Wireless Vital Signs Patch - Product Description
Neuro Event Labs Oy Pipeline Products & Ongoing Clinical Trials Overview
Nelli - Product Status
Nelli - Product Description
Neuro Event Labs Oy - Ongoing Clinical Trials Overview
Nelli - A Study of Detection of Paroxysmal Events Utilizing Computer Vision and Machine Learning
Northwestern University Pipeline Products & Ongoing Clinical Trials Overview
Wearable Device - Stroke Rehabilitation - Product Status
Wearable Device - Stroke Rehabilitation - Product Description
OBS Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview
Help4Mood System - Product Status
Help4Mood System - Product Description
Panasonic Corp Pipeline Products & Ongoing Clinical Trials Overview
Remote Sensing System - Product Status
Remote Sensing System - Product Description
PerGenix LLC Pipeline Products & Ongoing Clinical Trials Overview
PerGenix System - Product Status
PerGenix System - Product Description
Philips Healthcare Pipeline Products & Ongoing Clinical Trials Overview
Minicare H-2000 - Product Status
Minicare H-2000 - Product Description
Wearable Monitoring Device - COPD - Product Status
Wearable Monitoring Device - COPD - Product Description
Polytechnic University of Catalonia Pipeline Products & Ongoing Clinical Trials Overview
Cardio-Respiratory Monitoring Device - Product Status
Cardio-Respiratory Monitoring Device - Product Description
REMPARK Gait Guidance System - Product Status
REMPARK Gait Guidance System - Product Description
Profusa Inc Pipeline Products & Ongoing Clinical Trials Overview
Lumee Oxygen Sensing System - Product Status
Lumee Oxygen Sensing System - Product Description
Profusa Inc - Ongoing Clinical Trials Overview
Lumee Oxygen Sensing System - A Study to Explore the Use of Lumee Oxygen Platform in CLTI Patients and to Characterize Its Diagnostic Value: OMNIA
Lumee Oxygen Sensing System - Effectiveness of Measuring Local Tissue Oxygen in Response to Induced Hemodynamic Changes with the Profusa's Wireless Lumee Oxygen Platform in Patients with Peripheral Artery Disease (PAD)
Rethink Medical Inc Pipeline Products & Ongoing Clinical Trials Overview
CorBand - Product Status
CorBand - Product Description
Rowan University Pipeline Products & Ongoing Clinical Trials Overview
Non-Invasive Wearable Sensor - Product Status
Non-Invasive Wearable Sensor - Product Description
Sensaris Pipeline Products & Ongoing Clinical Trials Overview
SensPack - Product Status
SensPack - Product Description
Sensogo Ltd. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
SensoGo System - Product Status
SensoGo System - Product Description
Seoul National University Hospital Pipeline Products & Ongoing Clinical Trials Overview
Wearable Heart Rate Monitoring Device - Product Status
Wearable Heart Rate Monitoring Device - Product Description
Sister Kenny Research Center Pipeline Products & Ongoing Clinical Trials Overview
3-WAM - Product Status
3-WAM - Product Description
SomnoPatch Home Sleep Lab Pipeline Products & Ongoing Clinical Trials Overview
SomnoPatch - Product Status
SomnoPatch - Product Description
SPO Global Inc Pipeline Products & Ongoing Clinical Trials Overview
SPO Sports Watch - Product Status
SPO Sports Watch - Product Description
University of California Berkeley Pipeline Products & Ongoing Clinical Trials Overview
Wearable Sweat Sensor - Product Status
Wearable Sweat Sensor - Product Description
University of California Los Angeles Pipeline Products & Ongoing Clinical Trials Overview
Sleep Monitoring System - Product Status
Sleep Monitoring System - Product Description
Wireless Remote Sensing Device - Product Status
Wireless Remote Sensing Device - Product Description
University of Colorado Pipeline Products & Ongoing Clinical Trials Overview
Activity Monitor - Product Status
Activity Monitor - Product Description
University of Illinois at Chicago Pipeline Products & Ongoing Clinical Trials Overview
Physical Activity Monitoring Device - Product Status
Physical Activity Monitoring Device - Product Description
University of Michigan Pediatric Device Consortium Pipeline Products & Ongoing Clinical Trials Overview
Pediatric Motion Detection Device - Product Status
Pediatric Motion Detection Device - Product Description
University of Missouri Pipeline Products & Ongoing Clinical Trials Overview
Motion Tracker - Product Status
Motion Tracker - Product Description
University of New Mexico Pipeline Products & Ongoing Clinical Trials Overview
Wireless Medication Monitor - Product Status
Wireless Medication Monitor - Product Description
University of Toronto Pipeline Products & Ongoing Clinical Trials Overview
Telemonitoring System - Product Status
Telemonitoring System - Product Description
University of Utah Pipeline Products & Ongoing Clinical Trials Overview
Wearable Sensor Patch - Heart Failure Prediction - Product Status
Wearable Sensor Patch - Heart Failure Prediction - Product Description
Vitls Inc Pipeline Products & Ongoing Clinical Trials Overview
Vitls Platform - Respiration Rate - Product Status
Vitls Platform - Respiration Rate - Product Description
Vitls Platform - SpO2 - Product Status
Vitls Platform - SpO2 - Product Description
Vivonics Inc Pipeline Products & Ongoing Clinical Trials Overview
Aware - Product Status
Aware - Product Description
Waldo Health Pipeline Products & Ongoing Clinical Trials Overview
Waldo Telecardiology System - Product Status
Waldo Telecardiology System - Product Description
Wave Technology Group, Inc. (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
Wave EEG Monitor - Product Status
Wave EEG Monitor - Product Description
Xhale Inc Pipeline Products & Ongoing Clinical Trials Overview
Assurance PCA Guardian - Product Status
Assurance PCA Guardian - Product Description
Glossary
+ 1 888 391 5441
sales@reportsandreports.com
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release External Remote Patient Monitoring Devices Market Report- Growth, Share and Analysis of Key Players: ATLASense Biomed Ltd, Barron Associates here
News-ID: 2447220 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
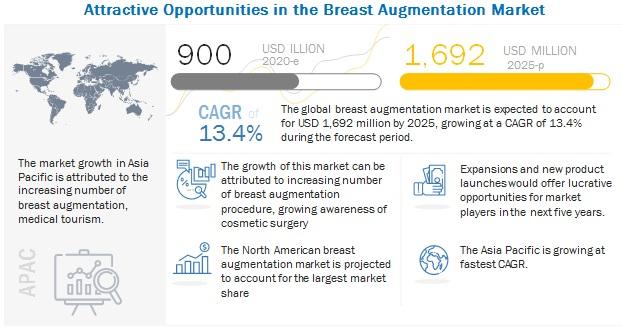
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Product
Product Launch
CHENNAI, INDIA - ShiningBot Data Analytics Private Limited, a leader in consumer behavior intelligence, today announced the official launch of ShiningBot version 2.0, a cloud-based platform designed to turn standard Guest WiFi into a sophisticated "intelligence layer" for physical businesses.
In an era where brick-and-mortar establishments struggle to match the data-rich insights of e-commerce, ShiningBot bridges the gap. By leveraging existing WiFi infrastructure, the platform allows Shopping Malls, Hotels, Hospitals, and…
Genstore Ranks #1 Product of the Day on Product Hunt
Los Angeles - September 11, 2025 - Genstore [https://www.genstore.ai/], an AI-native e-commerce platform, ranked #1 Product of the Day on Product Hunt and emerged as one of the week's top-trending products. The recognition underscores strong community support for Genstore's mission to make advanced commerce simple, accessible, and cost-efficient for small and medium-sized businesses worldwide.
Image: https://www.globalnewslines.com/uploads/2025/09/ab03aa9cb9a17e4c42e998d53f216bde.jpg
"Genstore lets anyone start selling online with just a prompt. But of course, that's just the…
Large Volume Parenteral Product Market New Product Development & Latest Trends
The global Large Volume Parenteral (LVP) market is poised for significant growth, projected to reach a value of approximately $12.5 billion in 2024. During the forecast period from 2025 to 2034, the market is expected to expand at a robust Compound Annual Growth Rate (CAGR) of 6.5%, culminating in an estimated market value of $22 billion by 2034.
Exactitude Consultancy., Ltd. released a research report offers a comprehensive examination of the…
Product technology, product usage tips, industry trends
Product Craftsmanship: Yiwu LABON Stationery Co., Ltd. Showcases Superior Craftsmanship in OEM Notebooks
Yiwu LABON Stationery Co., Ltd., established in 2003, has built a reputation for exceptional craftsmanship in the OEM notebook industry. Our factory-based company combines traditional techniques with modern innovation to create notebooks that stand out for their quality and design. Each notebook crafted by Yiwu LABON represents a meticulous process where attention to detail and precision are paramount.…
Product List: The Ultimate Destination for Product and Deal Discovery
Finding the right product or tool to suit your needs can be a daunting task, and securing the best deal on them can be equally challenging.
Each day, plenty of tools are launched, each with unique use cases. Individuals across various industries can benefit from these tools as they simplify their tasks compared to traditional methods. However, it's essential to consider the cost, as some tools are free while others come…
Logistics Packaging Market Enhance Product Safety, Maintain Product Quality, Ext …
MarketResearchReports.Biz presents this most up-to-date research on "Logistics Packaging Market: Global Industry Analysis 2013-2017 and Opportunity Assessment 2018-2028"
The global logistics sector continues to develop at an impressive rate. As a result, the packaging industry is undergoing enormous changes with specified focus on posing innovative packaging tools/products to various industry verticals. Logistics packaging is primarily done to enhance product safety, maintain product quality, extended product storage, and cater to other aspects…