Press release
Global Chemotherapy Induced Peripheral Neuropathy Pipeline Market Key Players Analysis MediciNova, Asahi Kasei Pharma Corp., AnnJi Pharmaceutical, EA Pharma, Aptinyx, Sonnet Biotherapeutics Holdings, Toray, Solasia Pharma and Others
Chemotherapy Induced Peripheral Neuropathy Pipeline Market Insight, 2021 report provides comprehensive insights about 13+ companies and 13+ pipeline drugs in Chemotherapy Induced Peripheral Neuropathy pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Geography Covered
- Global coverage
Get a Free Sample Copy of Global Chemotherapy Induced Peripheral Neuropathy Pipeline Market Research Report at https://www.reportsnreports.com/contacts/requestsample.aspx?name=4706879
Chemotherapy Induced Peripheral Neuropathy Understanding
Chemotherapy Induced Peripheral Neuropathy: Overview
Chemotherapy-induced painful neuropathy (CIPN) is a predominantly sensory neuropathy that may be accompanied by motor and autonomic changes. CIPN is a major dose-limiting side effect of several first-line chemotherapeutic agents. The symptoms of CIPN include tingling, pain, decreased sensation, increased sensitivity to touch, temperature, pressure, pain; muscle weakness, and/or irreversible nerve damage. Diagnosis of CIPN is based on history, clinical examination and supporting laboratory investigations. These include electromyography with nerve conduction studies, skin biopsies to evaluate cutaneous nerve innervation, and nerve and muscle biopsies for histopathological evaluation. Treatment of CIPN depends on discontinuation or lowering the dose of the anti-cancer drug. Persistent neuropathic pain can be treated with anti-seizure medications, antidepressants, or analgesics including drugs. Commonly used medications include Lyrica, Neurontin, Cymbalta, Celebrex, Elavil, and Lipoderm Patch. Regular exercise, occupational and physical therapy, reducing alcohol use, and treating preexisting medical conditions (vitamin B12 deficiency) may reduce the risk of CIPN.
"Chemotherapy Induced Peripheral Neuropathy - Pipeline Insight, 2021" report outlays comprehensive insights of present scenario and growth prospects across the indication. A detailed picture of the Chemotherapy Induced Peripheral Neuropathy pipeline landscape is provided which includes the disease overview and Chemotherapy Induced Peripheral Neuropathy treatment guidelines. The assessment part of the report embraces, in depth Chemotherapy Induced Peripheral Neuropathy commercial assessment and clinical assessment of the pipeline products under development. In the report, detailed description of the drug is given which includes mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, Chemotherapy Induced Peripheral Neuropathy collaborations, licensing, mergers and acquisition, funding, designations and other product related details.
Get a 20% and More Discount on Global Chemotherapy Induced Peripheral Neuropathy Pipeline Market Research Report at https://www.reportsnreports.com/contacts/discount.aspx?name=4706879
Report Highlights
- The companies and academics are working to assess challenges and seek opportunities that could influence Chemotherapy Induced Peripheral Neuropathy R&D. The therapies under development are focused on novel approaches to treat/improve Chemotherapy Induced Peripheral Neuropathy.
Chemotherapy Induced Peripheral Neuropathy Emerging Drugs Chapters
This segment of the Chemotherapy Induced Peripheral Neuropathy report encloses its detailed analysis of various drugs in different stages of clinical development, including phase III, II, I, preclinical and Discovery. It also helps to understand clinical trial details, expressive pharmacological action, agreements and collaborations, and the latest news and press releases.
Chemotherapy Induced Peripheral Neuropathy Emerging Drugs
- MN-166 (ibudilast): MediciNova
MN-166 (ibudilast) is a first-in-class, orally bioavailable, small molecule macrophage migration inhibitory factor inhibitor and phosphodiesterase (PDE) -4 and -10 inhibitor that suppresses pro-inflammatory cytokines and promotes neurotrophic factors. MN-166 (ibudilast)'s anti-neuroinflammatory and neuroprotective actions have been demonstrated in preclinical and clinical studies and the molecule is currently under Phase II development for the treatment of chemotherapy induced peripheral neuropathy (CIPN).
- ART-123: Asahi Kasei Pharma Corp.
ART-123, a recombinant human soluble thrombomodulin composed of the extracellular domain of thrombomodulin. The drug is in Phase II clinical studies for the treatment of chemotherapy induced peripheral neuropathy, and solid tumors. In 2008, ART-123 was approved for the treatment of disseminated intravascular coagulation (DIC) in Japan.
Further product details are provided in the report ..
Chemotherapy Induced Peripheral Neuropathy: Therapeutic Assessment
This segment of the report provides insights about the different Chemotherapy Induced Peripheral Neuropathy drugs segregated based on following parameters that define the scope of the report, such as:
- Major Players in Chemotherapy Induced Peripheral Neuropathy
There are approx. 13+ key companies which are developing the therapies for Chemotherapy Induced Peripheral Neuropathy. The companies which have their Chemotherapy Induced Peripheral Neuropathy drug candidates in the most advanced stage, i.e. Phase II include, MediciNova.
For More Details Inquire at https://www.reportsnreports.com/contacts/inquirybeforebuy.aspx?name=4706879
- Phases
The report covers around 13+ products under different phases of clinical development like
- Late stage products (Phase III)
- Mid-stage products (Phase II)
- Early-stage product (Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
- Route of Administration
Chemotherapy Induced Peripheral Neuropathy pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
- Oral
- Parenteral
- Intravitreal
- Subretinal
- Topical
- Molecule Type
Products have been categorized under various Molecule types such as
- Monoclonal Antibody
- Peptides
- Polymer
- Small molecule
- Gene therapy
- Product Type
Drugs have been categorized under various product types like Mono, Combination and Mono/Combination.
Chemotherapy Induced Peripheral Neuropathy: Pipeline Development Activities
The report provides insights into different therapeutic candidates in phase III, II, I, preclinical and discovery stage. It also analyses Chemotherapy Induced Peripheral Neuropathy therapeutic drugs key players involved in developing key drugs.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing along with a thorough therapeutic assessment of emerging Chemotherapy Induced Peripheral Neuropathy drugs.
Chemotherapy Induced Peripheral Neuropathy Report Insights
- Chemotherapy Induced Peripheral Neuropathy Pipeline Analysis
- Therapeutic Assessment
- Unmet Needs
- Impact of Drugs
Chemotherapy Induced Peripheral Neuropathy Report Assessment
- Pipeline Product Profiles
- Therapeutic Assessment
- Pipeline Assessment
- Inactive drugs assessment
- Unmet Needs
Direct Purchase of Global Chemotherapy Induced Peripheral Neuropathy Pipeline Market Research Report at https://www.reportsnreports.com/purchase.aspx?name=4706879
Key Questions
Current Treatment Scenario and Emerging Therapies:
- How many companies are developing Chemotherapy Induced Peripheral Neuropathy drugs?
- How many Chemotherapy Induced Peripheral Neuropathy drugs are developed by each company?
- How many emerging drugs are in mid-stage, and late-stage of development for the treatment of Chemotherapy Induced Peripheral Neuropathy?
- What are the key collaborations (Industry-Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Chemotherapy Induced Peripheral Neuropathy therapeutics?
- What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Chemotherapy Induced Peripheral Neuropathy and their status?
- What are the key designations that have been granted to the emerging drugs?
Key Players
- MediciNova
- Asahi Kasei Pharma Corp.
- AnnJi Pharmaceutical
- EA Pharma
- Aptinyx
- Sonnet Biotherapeutics Holdings
- Toray
- Solasia Pharma
- Enveric Biosciences
- Regenacy Pharmaceuticals
- NeuroBo Pharmaceuticals
Key Products
- MN-166
- ART-123
- AJ302
- EA-4017
- NYX-2925
- Recombinant Interleukin-6
- TRK-750
- SP-04
- Research programme: therapies Enveric Biosciences
- Ricolinostat
- NB-01
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets.
+ 1 888 391 5441
sales@reportsandreports.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Chemotherapy Induced Peripheral Neuropathy Pipeline Market Key Players Analysis MediciNova, Asahi Kasei Pharma Corp., AnnJi Pharmaceutical, EA Pharma, Aptinyx, Sonnet Biotherapeutics Holdings, Toray, Solasia Pharma and Others here
News-ID: 2384732 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
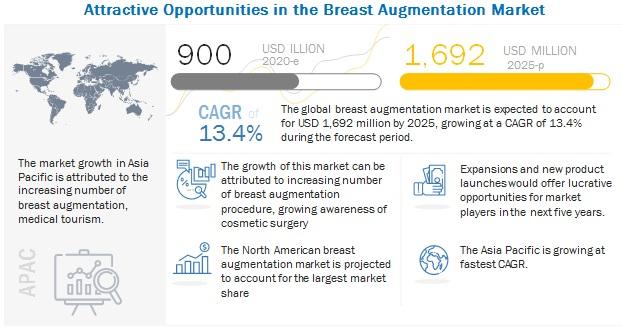
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Chemotherapy
Chemotherapy Devices Market - Innovation for Life: Chemotherapy Devices Driving …
Newark, New Castle, USA: The "Chemotherapy Devices Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Chemotherapy Devices Market: https://www.growthplusreports.com/report/chemotherapy-devices-market/8623
This latest report researches the industry structure, sales, revenue,…
Chemotherapy Devices Market 2022 Will Boom With Advanced Chemotherapy Technologi …
New Research Study ""Chemotherapy Devices Market 2022 analysis by Market Trends (Drivers, Constraints, Opportunities, Threats, Challenges and Investment Opportunities), Size, Share and Outlook"" has been added to Coherent Market insight
The most recent Global Chemotherapy Devices Market report includes a high-level overview of the industry as well as in-depth analysis of key areas. The overview presented highlights the definition of products and services, as well as their associated applications, at the…
Cancer, chemotherapy and its treatment
Chemotherapy is one of the most commonly used cancer therapies. It employs the use of certain medications to destroy cancer cells or prevent them from spreading to other parts of the body. Chemotherapy may be prescribed alone or in combination with surgery or radiation therapy. Along with chemotherapy, you may also take newer types of cancer-fighting medications.
Chemotherapy can be taken as pills or as injections. You may visit a clinic…
Chemotherapy induced Nausea and Vomiting (CINV) TreatmentS Market Driven by Risi …
According to WHO cancer is one of the most leading causes for death with around 8 million deaths and 14 million new patients in 2012. Chemotherapy, which is received by approximately four million cancer patients each year, is among the most commonly used treatment to fight cancer. However, this mode of treatment has many side effects, an estimate of around 70% - 80% of the patients receiving chemotherapy treatment experience…
Nano Chemotherapy Market Nano Chemotherapy Clinical Pipeline Report 2022
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
Nano Chemotherapeutics Mediations andndash; Solicitation to Nanomedicine
1.1 Overview
1.2 Nano Chemotherapeutics with Pharmaceutical Potential
Significance of Nano Chemotherapeutics to overcome the Chemotherapy Limitations
2.1 Limitations of Conventional Chemotherapy
2.2 Nanotechnology in Cancer Targeting
2.2.1 Active Targeting
2.2.2 Passive Targeting
2.2.3 pH Dependent Drug Delivery Through Nanoparticles
Role…
Nano Chemotherapy Is Emerging As An Important Anti-Cancer Modality By Supplement …
“Global Nano Chemotherapy Market and Clinical Trials Outlook 2022” report highlights the current development in the in the field of nano chemotherapy. Report gives comprehensive insight on various clinical and non-clinical parameters associated with the expansion of global nano chemotherapeutics market. The clinical and pricing insight on chemotherapeutics nanoformulations of approved drugs helps to understand the current market scenario of the nano chemotherapeutics.
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Download Report:…