Press release
eCOA, eSource & Clinical Trials Market Demand, Application, Key-Players, Growth Analysis and Forecast to 2029 | OpenClinica LLC, CRF Health Inc, ERT Clinical, Medldata Solutions, Inc, ArisGlobal LLC
The latest Fact.MR study indicates healthcare facilities, contract research organizations, and educational & research institutes collectively spent ~US$ 1,950 Mn on eCOA, eSource and clinical trials solutions in 2018. The eCOA, eSource & clinical trials market is envisaged to witness substantial traction through 2019, with gains primarily driven by the growing penetration of digitization in healthcare sector and substantial increase in pharma spending on clinical trials.Rapidly expanding deployment of clinical trial solutions, which contributed to ~40% of the total spending on eCOA, eSource, and clinical trials in 2018, will continue to appeal a widening bandwidth of institutions through 2029, says the report.
Spending on eCOA Set to Quadruple
The Fact.MR study opines that though the deployment of clinical trial solutions overshadowed the use of Electronic Clinical Outcome Assessment (eCOA), eSource, and EDA in 2018, the market is envisaged to witness a range of changes during the foreseeable period. Growing penetration of Bring Your Own Device (BYOD) trend in clinical trials is highly likely to pace up the use of eCOAs, such as ePROS, ClinROs, ObsROs, and PerfORs across wide range of pharmaceutical companies and research facilities.
To remain ‘ahead’ of your competitors, request a sample: https://www.factmr.com/connectus/sample?flag=S&rep_id=4042
eCOAs is envisaged to hold ~45% of the overall spending share by the end of 2029, in line with the growing use of smartphones and tablets to seamlessly collect clinical data for analysis. Additionally, reduced cost and high scalability of eCOA are the primary determinants that continue to push its adoption during clinical trials, which is envisaged to contribute significantly to the growth of the eCOA, eSource & clinical trials market
Stakeholders Eyeing Opportunities through Collaborations
As the healthcare sector delves deeper into advanced technology, a large number of companies have formulated unique strategies to remain unaffected by the curve of change. Several pharmaceutical companies, research organizations and CROs have placed their focus on collaborations, while they embrace digitization to create patient-centric experiences. For instance, Signant Health has launched a partner program to collaborate with Clinical Research Organizations (CROs) that are dedicated towards improving clinical trials using patient-centric technology.
Additionally, Iqvia has recently launched a novel eCOA cloud based technology platform to quantify the patient experience, while increasing efficiency and reducing timelines at the same time.
Growing Demand for Clinical Trials to Underpin Spending of Emerging Regions
As per the Fact.MR study, the increasing prevalence of various chronic infectious diseases has led to a dire unmet need for efficient clinical trials in developing countries. Moreover the rising medical expense in these regions, which can be attributed to the fact that private hospitals are the key healthcare providers herein, continue to fuel clinical trial participation in developing countries. This, coupled with the growing awareness about the cost and efficiency benefits of using eCOA, eSource & clinical trial solutions for conducting clinic trials, and comparatively lenient regulatory framework are likely to add to the lucrativeness of developing countries, such as China and India for market players.
Company profiles includes are, OpenClinica LLC, CRF Health Inc, ERT Clinical, Medldata Solutions, Inc, ArisGlobal LLC, HealthDiary Inc, ICON Plc, PAREXEL International Corporation, OmicComm Systems Inc, Medrio Inc, Medable, Oracle Corporation, Medspace Holdings Inc, Covancce Inc, and Bio-Optronics Inc.
Developed Regions Spearhead in Clinical Trials Deployment Rate
The rapidly advancing healthcare infrastructure, fast internet connectivity, and stringent regulatory product approval framework continue to make North America and Europe a hotbed of opportunities for stakeholders. With an increased number of companies getting cloud based (SaaS) deployment of clinical trial solutions in developed regions, owing to smooth internet connectivity, the market continues to accelerate at a steady pace. Cloud based deployment of digitized solutions accounted for 64% of the overall spending in Europe.
For critical insights on this market, request for Methodology here: https://www.factmr.com/connectus/sample?flag=RM&rep_id=4042
As per the study North America eCOA, eSource & clinical trials spending has been witnessing a steady growth over the years, with the US spending expected to account for ~90% revenue share in the regional eCOA, eSource & clinical trials spending in 2018. In view of the manifold cost and processing benefits of digitization in clinical trials, a larger number of pharmaceutical companies, contract research organizations, and education & research institutions have shifted their focus on paperless approaches. Growing adoption of clinical trial solutions, which contributed ~40% revenue share in North America eCOA, eSource, and clinical trials market, continue to create a window of opportunities of growth for market players.
Contact:
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E: sales@factmr.com
Corporate Headquarter:
Unit No: AU-01-H Gold Tower (AU),
Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers,
Dubai, United Arab Emirates
About Fact.MR
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions. While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise. Spanning a wide range – from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed. Our sales offices in United States and Dublin, Ireland. Headquarter based in Dubai, UAE. Reach out to us with your goals, and we’ll be an able research partner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release eCOA, eSource & Clinical Trials Market Demand, Application, Key-Players, Growth Analysis and Forecast to 2029 | OpenClinica LLC, CRF Health Inc, ERT Clinical, Medldata Solutions, Inc, ArisGlobal LLC here
News-ID: 2235234 • Views: …
More Releases from Fact.MR
Bottled Water Market Valuation, ROI Potential & Long-Term Growth Prospects (2026 …
The global bottled water market is undergoing a profound structural evolution, with its valuation projected to rise from USD 320 billion in 2026 to USD 521.2 billion by 2036. According to specialized industry analysis, the market is set to expand at a compound annual growth rate (CAGR) of 5.0%, driven by a global shift away from sugary carbonated drinks and the rising necessity for safe, accessible drinking water in rapidly…

Gamma Probe Devices Market Dynamics 2026-2036: Risk Assessment, Supply Chain Ins …
The global Rare Neurological Disease Treatment Market is expected to register substantial growth over the coming decade, underpinned by rising disease prevalence, expanding diagnostic capabilities, and accelerating research and development of targeted therapies. Industry analysis indicates that the market, valued at approximately USD 6.3 billion in 2025, is projected to reach around USD 12.8 billion by 2035, representing a compound annual growth rate (CAGR) of about 7.2% over the forecast…

Rare Neurological Disease Treatment MMarket: Portfolio Priorities, Adoption Tren …
The global Rare Neurological Disease Treatment Market is expected to register substantial growth over the coming decade, underpinned by rising disease prevalence, expanding diagnostic capabilities, and accelerating research and development of targeted therapies. Industry analysis indicates that the market, valued at approximately USD 6.3 billion in 2025, is projected to reach around USD 12.8 billion by 2035, representing a compound annual growth rate (CAGR) of about 7.2% over the forecast…
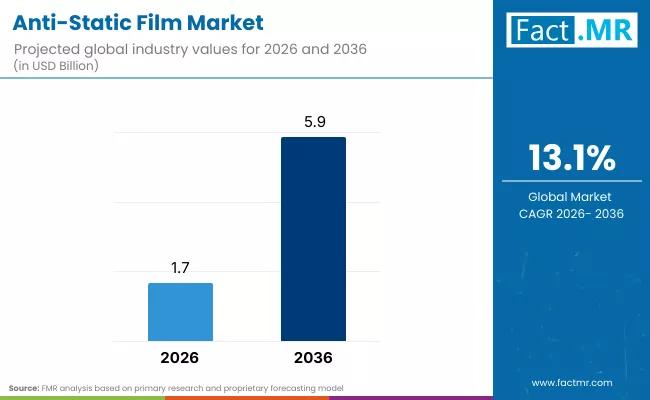
Anti-Static Film Market Size, Share & Forecast: High-Growth Segments, Value Chai …
The global anti-static film market is entering a decade of sustained, technology-driven expansion, with its valuation projected to rise from USD 730 million in 2026 to USD 1.02 billion by 2036. According to specialized industry analysis, the market is set to expand at a compound annual growth rate (CAGR) of 8.8%, fueled by the explosive growth of global e-commerce and the transition toward more sensitive, sub-nanometer semiconductor architectures that require…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…