Press release
New Application Note on the Differential Lysis to Provide Autosomal DNA Profiles in Forensics
A novel economic and universally applicable approach constitutes the mini spin-column format. Sampletype i-sep® DL has the potential to decrease the forensic DNA analysis backlog of sexual assault cases by circumventing time-consuming conventional differential extraction procedure. Numerous gradual extractions in the same device allow an immunological pre-test using the identical sample. Thus improves all over efficiency and ensures a subsequent differential lysis will be meaningful.Dresden, Germany, May 10, 2012 -- Biotype Diagnostic GmbH, a developer and marketer of innovative products for analysis of DNA especially in the field of clinical research and diagnostic, today announced publication of an application note showing that its Sampletype i-sep® DL has the potential to decrease the forensic DNA analysis backlog of sexual assault cases by circumventing time-consuming conventional differential extraction procedure.
A novel economic and universally applicable approach constitutes the mini spin-column format Sampletype i-sep® DL. This extraction unit contains a unique self-sealing filter compartment that prevents flow-through of different kinds of liquid during incubation. Only centrifugation allows DNA, released during sample lysis, to be quantitatively transferred into the collection tube. No extra sample carrier transfer and no extra pipetting steps are necessary. Furthermore, numerous gradual extractions in the same device allow an immunological pre-test using the identical sample. Thus improves all over efficiency and ensures a subsequent differential lysis will be meaningful.
The extracted DNA can be used to produce accurate, reliable and reproducible autosomal DNA profiles of both victim and perpetrator for forensic casework analysis. The system is based on a single tube assembly and is easy to use, yielding high quality DNA in less time than conventional methods.
"Our Sampletype i-sep® extraction system illustrates how this novel extraction system enables scientists and practitioners to obtain accurate, reliable and reproducible DNA from a variety of difficult samples rapidly, simply and cost effectively," commented Dr. Helge Schnerr, Head of Product Management at Biotype Diagnostic. "The single tube assembly describes an alternative to, rather than automation of, the current methods as a means of reducing analysis time without compromising extraction efficiency, purity of product, sensitivity, or selectivity of the sample preparation process,” he added.
The results included in this application note show that Sampletype i-sep® DL dramatically rationalizes the process of differential lysis. Biotype’s approach significantly reduces the time and cost of DNA extractions while simplifying laboratory workflow, minimizing error, reducing the risk of contamination, ensuring sample integrity and facilitating extractions from trace samples. After the manual preparation of the individual DNA extracts, lysates can be processed further manual or on any laboratory automation system. The product is ethylene oxide sterilized and the technology has been validated for use in highly regulated applications, including forensic analysis.
For more information on the product, visit www.sampletype.com or email info@biotype.de. The application note, titled "Differential lysis in a single-tube extraction process for accurate forensic profiling" was prepared with support of the State Office of Criminal Investigation Saxony. It is also available at www.nature.com/app_notes/nmeth/2012/120805/pdf/an8507.pdf.
Biotype profile:
Biotype Diagnostic is an innovative and dynamic company having long lasting experiences in developing and manufacturing molecular DNA-analytics. In close collaboration with scientific experts the company transfers innovative product-ideas into valuable applications of premium quality. The product portfolio spans medical applications for haematology/oncology and dermatology but also includes DNA-extraction systems for challenging sample preparation.
Sampletype i-sep® DL an economic and universally applicable approach to prepare particle-free DNA extracts from mixed specimens
Biotype Diagnostic is an innovative and dynamic company having long lasting experiences in developing and manufacturing molecular DNA-analytics. In close collaboration with scientific experts the company transfers innovative product-ideas into valuable applications of premium quality. The product portfolio spans medical applications for haematology/oncology and dermatology but also includes DNA-extraction systems for challenging sample preparation.
Contact:
Dr. Helge Schnerr
Biotype Diagnostic GmbH
Moritzburger Weg 67
Dresden, Germany 01109
+49 351 8838 440
h.schnerr@biotype.de
http://www.biotype.de
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release New Application Note on the Differential Lysis to Provide Autosomal DNA Profiles in Forensics here
News-ID: 220904 • Views: …
More Releases from Biotype Diagnostic GmbH

New dynamics in monitoring diagnostics in leukemia
Dresden/Germany
Diagnosis leukemia and stem cell transplantation
The diagnosis "leukemia" for patients means to undergo several stressful therapeutic interventions. If chemotherapy is not effective, stem cell transplantation mostly is the last chance for healing. However, a sudden graft rejection or disease relapse can always occur despite the successful transfer of the hematopoietic stem cells from donor to recipient. Therefore, the regular quantitative assessment of the donor and recipient cell-portion in the blood…
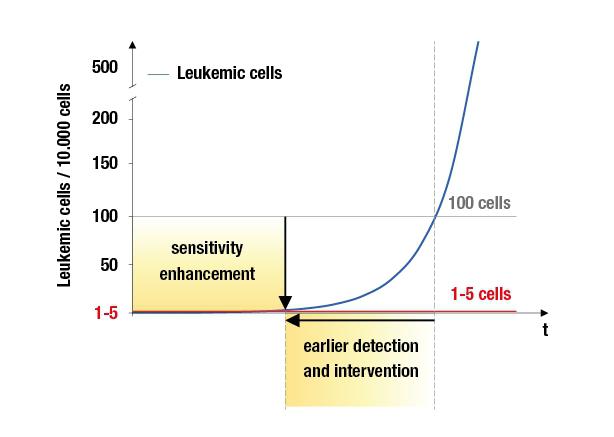
Can quantitative chimerism monitoring serve as surrogate marker to predict clini …
A promising method indicates success
Allogeneic stem cell transplantation (HSCT) is an effective therapy for hematopoietic disorders such as Acute Myeloid Leukemia (AML) or the Myelodysplastic Syndrome (MDS). However, imminent relapse remains a serious problem. Routinely, monitoring of minimal residual disease (MRD) by diseases-specific markers such as FLT3, NPM1, BCR-ABL, AML-ETO and others is performed to signify threatening relapse as early as possible. However, a high number of patients lack…
Summer is Holiday Time and Fungal Infections are Advancing Fast
Don’t miss out. A multiparameter PCR-analysis for differential diagnostics of dermatophytosis (also ringworm, athletes foot) will become available as full in-vitro-diagnostics.
Dresden, Germany, July 18, 2012 -- Today, Biotype set the 1st of August as date, on which its latest product, Mentype® MycoDermQS, will be launched as full In-Vitro Diagnostics (IVD). On the recently held 18th ISHAM in Berlin, Germany, Biotype has initially introduced its multiparameter analysis. This product represents the…
More Releases for DNA
High-Quality Plasmid DNA Fuels Growth in Global DNA Plasmid Manufacturing Market
🌍 Market Overview
The DNA Plasmid Manufacturing Market is experiencing robust growth as advancements in cell & gene therapy, DNA vaccines, and genetic engineering continue to expand globally. Plasmid DNA plays a critical role as a raw material in the development of advanced therapies, fueling demand across biopharmaceutical research and production.
Key factors driving the market include:
Increasing adoption of gene and cell therapies
Rising prevalence of chronic and rare genetic disorders
Expansion of DNA-based…
DNA Synthesis Market Increasing Demand for Synthetic Genes and DNA Sequences
As demonstrated by Precision Business Insights (PBI), the latest report, the global DNA synthesis market was valued at USD 3,702.0 million in 2023 and is expected to reach USD 10,289.5 million by 2029, growing at a CAGR of 18.6% during the forecast period 2024-2030. The key drivers for the growth of the global DNA synthesis market include increasing demand for synthetic genes and DNA sequences, growing applications in the agriculture…
Wealth DNA Code Review Legit Price? (Wealth Manifestation DNA Code Audio Frequen …
Wealth DNA Code Wealth DNA Code is a digital program with seven minutes of soundtracks that manifest and listen to daily to activate the "Wealth DNA," which is part of your DNA to help you attract wealth by making money a part of your mentality and making your dreams to come true.
https://bit.ly/Visit-The-Official-Website-Here-To-Order-Wealth-DNA-Code
Making money, creating assets as well as increasing wealth are the primary objectives that every human being has to…
DNA Paternity Testing Market Size [2022-2029] -DNA Diagnostics Center, EasyDNA, …
A recent market research report added to repository of MR Accuracy Reports is an in-depth analysis of global DNA Paternity Testing. On the basis of historic growth analysis and current scenario of DNA Paternity Testing place, the report intends to offer actionable insights on global market growth projections. Authenticated data presented in report is based on findings of extensive primary and secondary research. Insights drawn from data serve as excellent…
DNA Paternity Testing Market Trends 2020 | Growth by Top Companies: DNA Diagnost …
The report begins with the overview of the DNA Paternity Testing Market and offers throughout development. It presents a comprehensive analysis of all the regional and major player segments that gives closer insights upon present market conditions and future market opportunities along with drivers, trending segments, consumer behaviour, pricing factors and market performance and estimation. The forecast market information, SWOT analysis, DNA Paternity Testing market scenario, and feasibility study are…
DNA Paternity Testing Market Rapidly Growing in Healthcare, Competitor Analysis …
The exclusive research report on the Global DNA Paternity Testing Market 2020 examines the market in detail along with focusing on significant market dynamics for the key players operating in the market. Global DNA Paternity Testing Industry research report offers granulated yet in-depth analysis of revenue share, market segments, revenue estimates and various regions across the globe.
Overview of Global DNA Paternity Testing Market:
This report studies the Global DNA Paternity Testing…