Press release
New study: Only reliable rapid corona tests make sense
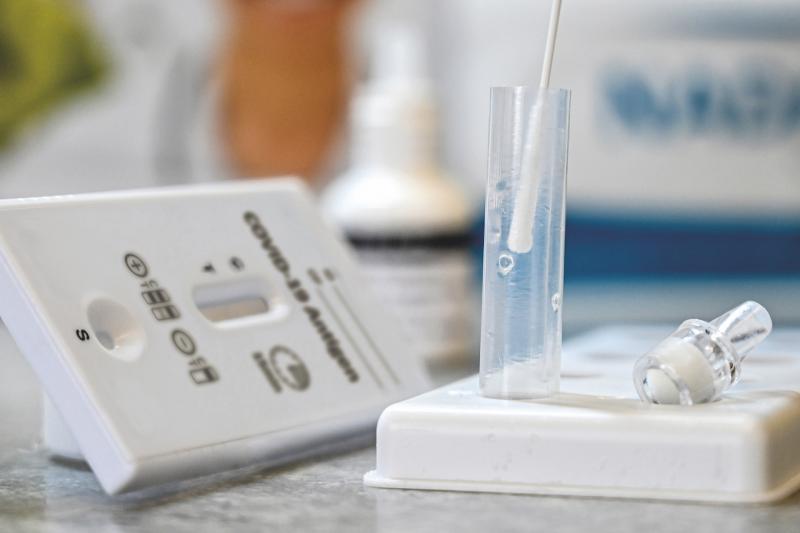
The NADAL® COVID-19 Ag rapid test from nal von minden GmbH was one of the best three tests evaluated
Corona rapid tests are playing an increasingly important role. In nursing homes, old people's homes and hospitals, they fulfil an especially important function: infectious people can be detected quickly and other people can be better protected against corona. Rapid tests are also very important at airports, schools, universities and at sporting events. The use of rapid tests could also shorten the quarantine period, which is often required when returning from risk areas.
But how reliable are the test results? Researchers at the Charité hospital in Berlin, led by virologist Prof. Christian Drosten, have now examined both the specificity and sensitivity of different tests. The specificity provides information on whether all healthy people who have been tested are recognised as healthy. The sensitivity indicates whether all sick people are also recognised as sick.
The NADAL® COVID-19 Ag rapid test from nal von minden GmbH was one of the best three tests evaluated: the specificity was 99.26 percent. In terms of sensitivity, the NADAL® COVID-19 Ag rapid test also scored outstandingly well in the Charité study. Roland Meißner, managing director of nal von minden GmbH: "We are pleased about the confirmation of the very good quality of our rapid test.“
"A total of 105 swab samples were analysed with the NADAL® COVID-19 Ag rapid test in the Charité study". The swabs were previously taken from the mouth or nasopharynx of patients, placed in a tube with liquid transport medium and frozen at minus 20 degrees Celsius until the examination. Subsequently, comparative tests were carried out in the laboratory in comparison to PCR.
The Charité's methodology differs slightly from clinical studies conducted by nal von minden GmbH. Tobias Roth, biochemist with a focus on virology: "We use the swabs directly on the antigen test. Charité, on the other hand, put the swabs in a liquid transport medium. As a result, the samples were diluted and the results determined by a different evaluation method".
The NADAL® COVID-19 Ag test can be performed directly on site and without additional equipment. The test detects specific viral components: These are so-called nucleoprotein antigens. These proteins coat the genetic material of SARS-CoV-2 consisting of ribonucleic acid (RNA). For comparison: In the PCR tests (PCR = polymerase chain reaction) used in the laboratory, the viral RNA is detected.
Hellwig PR, Gabriele Hellwig, Garstedter Weg 268, D- 22455 Hamburg, Tel.: +49 40 38 66 24 80, E-Mail: info@hellwig-pr.de, www.hellwig-pr.de
nal von minden GmbH, based in Moers, Germany, has been a specialist in the field of medical diagnostics for 38 years. Its portfolio includes rapid tests and laboratory tests for reliable diagnoses in the fields of bacteriology, cardiology, gynaecology, infectious diseases, urology and toxicology. The Covid-19 antigen rapid test is the latest development. nal von minden GmbH has a total of about 220 employees at 9 European locations.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release New study: Only reliable rapid corona tests make sense here
News-ID: 2202073 • Views: …
More Releases from nal von minden GmbH

Can a urine test act as an early warning system for a severe course of infection …
Acute kidney failure is one of the most dangerous consequences of a coronavirus infection. It’s no wonder that more and more patients are asking: What can I do to protect my kidneys? Above all, it is important to regularly monitor kidney function using means such as urine analysis. The Reactif urine tests from the medical-technology company nal von minden, which is based in Germany, determine up to 14 health and…
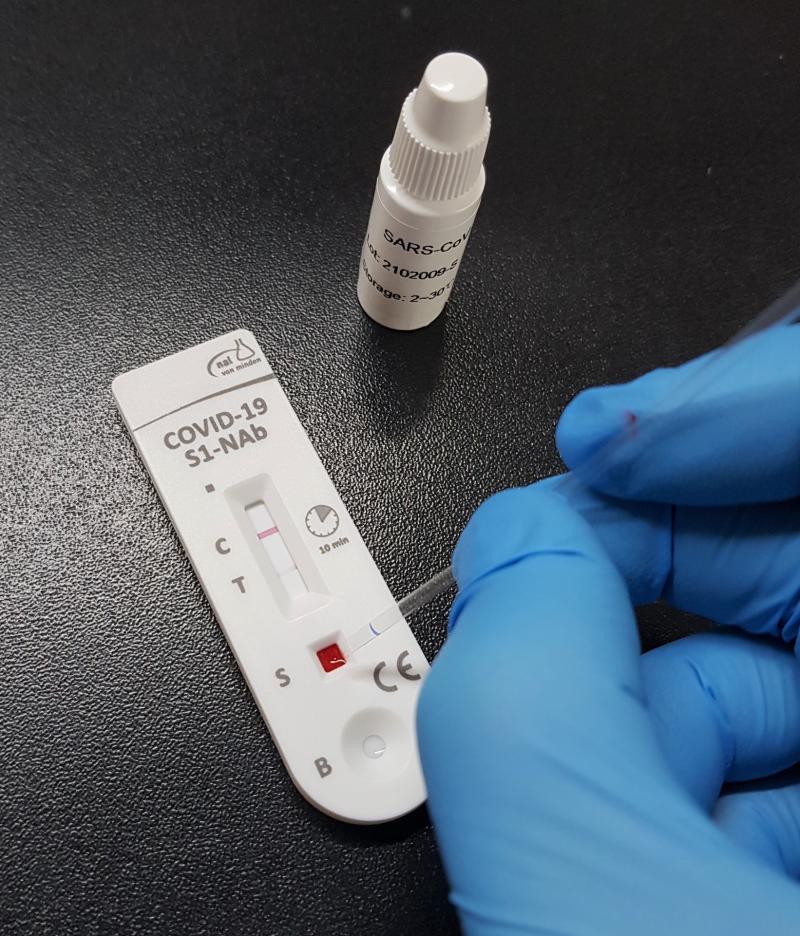
Is coronavirus still a danger, even if I’m vaccinated? A new rapid test needs …
Levels of uncertainty are running high – especially after having heard from the Standing Committee on Vaccinations (German: STIKO) that, despite being fully vaccinated, some people are nevertheless not protected from coronavirus. Antibody tests can now be offered to provide certainty on the presence of an individual’s immune response. nal von minden, a medical-technology company from Moers in Germany, has developed a rapid test that can reliably detect antibodies against…
nal von minden is calling for an extension to new EU regulations regarding in-vi …
nal von minden, a medical technology company from Germany, is calling for an extension to new EU regulations regarding in-vitro diagnostics. Because of the coronavirus, the EU’s original timetable cannot be adhered to. As it stands, all companies that sell in-vitro diagnostics such as rapid tests must re-register their products. This is because the new, stricter EU regulations for in-vitro diagnostics (IVDR) comes into force in May 2022.
“In the interest…
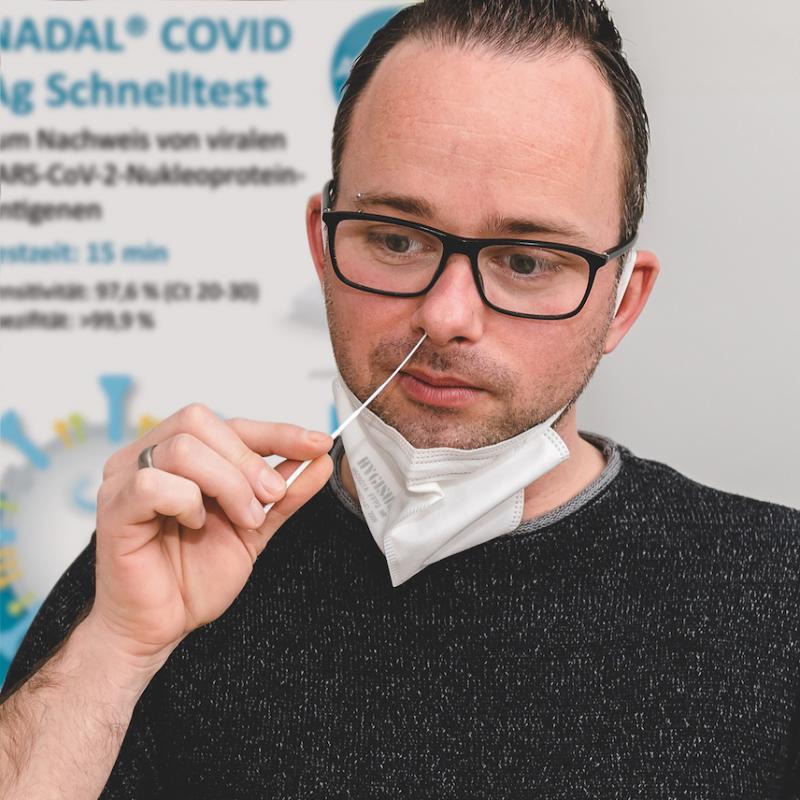
Only a swab from the anterior nasal passage is required
Coronavirus rapid tests from nal von minden GmbH (Germany), already well-reviewed and widely used, have undergone another stage of development. A swab taken from the anterior nasal passage is now all that is needed to carry out a test, rather than from the posterior nasal passage or oropharynx as was previously necessary. The NADAL® COVID-19 Ag Test is available all over Europe from the 22nd February.
“It’s much more comfortable when…
More Releases for NADAL®
Punch!® Software Releases Punch!CAD™ SharkCAD® & ViaCAD® 15
NOVATO, Calif., December 10, 2024 - Punch!® Software, a leading software developer of Home Design and CAD titles for over 25 years, today announced the release of new Windows and Mac versions of the SharkCAD® 15 and ViaCAD® 15 family of products.
This latest release encompasses a range of variants for the Windows and Macintosh platform: SharkCAD® Pro, SharkCAD®, ViaCAD® Pro, ViaCAD® 2D/3D, and ViaCAD® 2D.
Punch!CAD™ products continue to provide innovative…
Ultimate Chemicals® Featured in Manufacturing Marvels® on The Fox Business Net …
MOORE, OK., Oct. 8, 2021/ -- Ultimate Chemicals® Inc., an integrated manufacturer and supplier of chemicals and related services, is pleased to announce it will be showcased in a broadcast of Manufacturing Marvels® scheduled to air on October 14, 2021 at approximately 9:30-9:44 p.m. EST on The Fox Business Network® (FBN).
Ultimate Chemicals has created their products to help restore surfaces to their optimum surface capability. Non-destructive to the surfaces to…
Sunera Technologies, Inc Joins SAP® PartnerEdge® Delivering SAP® S/4HANA
Sunera Technologies, Inc Joins SAP® PartnerEdge® Delivering SAP® S/4HANA and Intelligent Enterprise to Manufacturing, Consumer Products, Higher education, and research, Industrial machinery and components, Life sciences and Automotive Industries
Chicago, IL, June 10, 2020 – Sunera Technologies, Inc announced that it had joined the SAP® PartnerEdge® program as a Re-sell & Services partner, through which it will resell SAP solutions to midsize organizations in North America. Through this engagement, SuneraTech…
Wimbledon Singles? Despite losing in the finals, Nadal and Sharapova still win t …
In a poll conducted by Mamboo, one of the fastest growing social dating websites in the world, tennis fans selected Nadal and Sharapova as the tennis stars they would most want to date.
“So basically as a consolation prize for coming in second at Wimbledon and to help them forget the finals, we can offer Nadal and Sharapova the opportunity to arrange as many dates as they could possibly want this summer…
American Megatrends Announces AMIBIOS®8 & Aptio®4.x Support for the Intel® Xe …
ATLANTA, GEORGIA - American Megatrends (AMI) is pleased to announce that its AMIBIOS®8 & Aptio®4.x products feature support for the new Intel® Xeon® 5500 Platform.
The Intel® Xeon® 5500 platform combines the Intel® Xeon® Processor 5500 Series with the Intel® 5520 Chipset for embedded, storage, security and communications infrastructure applications in a wide range of form factors. There are four different processor options for this platform with seven year lifecycle support…
CNUX .NET Providers for DB2® Earns IBM® DB2® Data Server Certification
CNUX Technologies Inc. announced today that CNUX .NET Providers for DB2® - Standard and Web Service Edition have achieved "Ready for IBM DB2 Data Server Software" status. With this designation CNUX customers can be assured that CNUX .NET Providers interacts and operates seamlessly with IBM® DB2® software.
The "Ready for IBM DB2 Data Server Software" program is a process designed for independent software vendors (ISVs) and solution integrators to validate their…