Press release
nal von minden is calling for an extension to new EU regulations regarding in-vitro diagnostics
nal von minden, a medical technology company from Germany, is calling for an extension to new EU regulations regarding in-vitro diagnostics. Because of the coronavirus, the EU’s original timetable cannot be adhered to. As it stands, all companies that sell in-vitro diagnostics such as rapid tests must re-register their products. This is because the new, stricter EU regulations for in-vitro diagnostics (IVDR) comes into force in May 2022.“In the interest of all citizens, we believe postponing the deadline for another year to be realistic”, says Dr Gerd Hagendorff, leader of the Quality Management department at nal von minden GmbH. “It would be ideal if the new regulations for in-vitro diagnostics came into effect in 2023, instead of May 2022.”
If not, a bottleneck can be expected with certain products, warns Dr. Hagendorff: “We manufacture 100 tests that are used, for example, in GP practices and hospitals. These include tests for heart attacks and tumour diagnoses. If these tests are no longer available in sufficient numbers, in a worst case scenario we could see delayed diagnoses and treatment.”
This doesn’t only affect nal von minden GmbH, but also many other small-to-medium sized companies, as well as larger firms in Germany and across Europe. “Despite the best efforts of manufacturers, the new IVDR cannot be implemented in the time we have left,” says Dr. Hagendorff.
The original EU timetable was already somewhat ambitious, criticises Dr. Hagendorff. The new regulations for in-vitro diagnostics came into effect EU-wide on 26th May 2017.The trigger was a scandal surrounding breast implants, and security for EU citizens was wanted in the field of medical technology. Since then, manufacturers have had 5 years to implement very time-consuming and labour-intensive changes.
However, even from the beginning, there were not enough so-called ‘notified bodies’ to carry out product testing and with sufficient lead time. “Unfortunately there are only 3 notified bodies available for all manufacturers across Europe who distribute in-vitro diagnostics,” explains Dr. Hagendorff. Even for the more-than 100 products from nal von minden GmbH which need to be IVDR registered, a best case scenario would require over 100 weeks, as the notified body estimates at least 1 week for each product. “This is almost impossible to accomplish by May 2022," he explains.
Due to the pandemic, the timetable cannot be adhered to anymore, says Dr. Hagendorff. “We have pooled our capacities in order to help contain the corona pandemic. We are producing millions of rapid tests every month, so there hasn’t been time to work on the EU regulations.”
“We are in favour of the new regulations, but not under strain and not at the expense of the population,” explains Dr Hagendorff. Furthermore, the same rules should apply to all companies. Last year, in direct response to the corona crisis, the transition period for medical devices according to MDR (Medical Device Regulation, e.g. for pacemakers) was extended by one year.
Hellwig PR
Gabriele Hellwig
Garstedter Weg 268
D- 22455 Hamburg
Tel.: +49 40 38 66 24 80
E-Mail: info@hellwig-pr.de
www.hellwig-pr.de
nal von minden GmbH, based in Moers, Germany, has been a specialist in the field of medical diagnostics for 38 years. Its portfolio includes rapid tests and laboratory tests for reliable diagnoses in the fields of bacteriology, cardiology, gynaecology, infectious diseases, urology and toxicology. nal von minden GmbH has a total of about 230 employees at 9 European locations. www.nal-vonminden.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release nal von minden is calling for an extension to new EU regulations regarding in-vitro diagnostics here
News-ID: 2278219 • Views: …
More Releases from nal von minden GmbH

Can a urine test act as an early warning system for a severe course of infection …
Acute kidney failure is one of the most dangerous consequences of a coronavirus infection. It’s no wonder that more and more patients are asking: What can I do to protect my kidneys? Above all, it is important to regularly monitor kidney function using means such as urine analysis. The Reactif urine tests from the medical-technology company nal von minden, which is based in Germany, determine up to 14 health and…
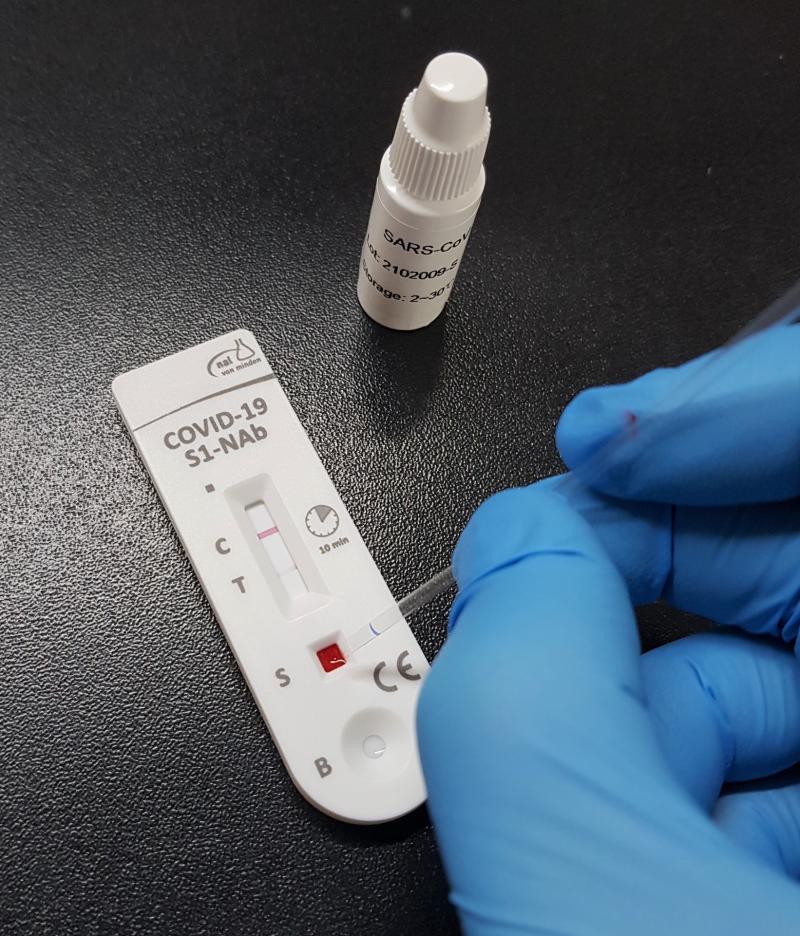
Is coronavirus still a danger, even if I’m vaccinated? A new rapid test needs …
Levels of uncertainty are running high – especially after having heard from the Standing Committee on Vaccinations (German: STIKO) that, despite being fully vaccinated, some people are nevertheless not protected from coronavirus. Antibody tests can now be offered to provide certainty on the presence of an individual’s immune response. nal von minden, a medical-technology company from Moers in Germany, has developed a rapid test that can reliably detect antibodies against…
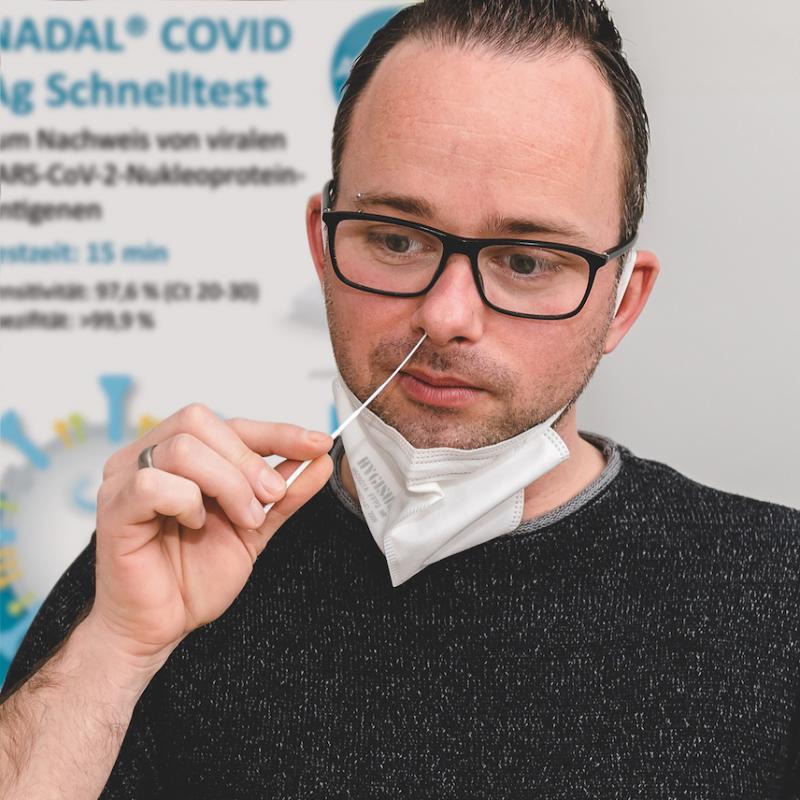
Only a swab from the anterior nasal passage is required
Coronavirus rapid tests from nal von minden GmbH (Germany), already well-reviewed and widely used, have undergone another stage of development. A swab taken from the anterior nasal passage is now all that is needed to carry out a test, rather than from the posterior nasal passage or oropharynx as was previously necessary. The NADAL® COVID-19 Ag Test is available all over Europe from the 22nd February.
“It’s much more comfortable when…
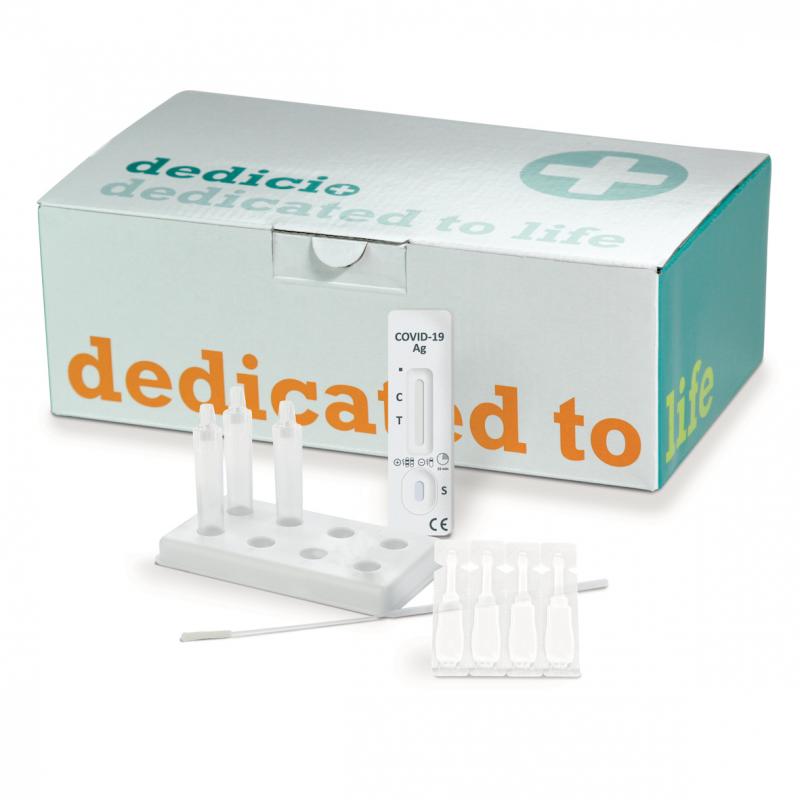
New rapid test for all students and teachers
In order to make it possible to attend school safely despite the coronavirus pandemic, the German medical technology firm nal von minden has developed a rapid test: The ‘dedicio® COVID-19 Antigen plus Test’ is easy to use and delivers reliable results in just 15 minutes. The new rapid test is already available.
“The new rapid test is designed to be a simple and easy-to-use disposable test”, says Roland Meißner, CEO at…