Press release
The medical device CRO market is estimated to be worth USD 15.7 billion in 2030, predicts Roots Analysis
Advances in the medical device industry have led to a substantial increase in developmental complexity, clinical trial conduct, and stringency of regulatory review, causing sponsors to rely on the technical and regulatory affairs management expertise of CROsRoots Analysis is pleased to announce the publication of its recent study, titled, “Medical Device CRO Market (2nd Edition), 2020-2030”.
For more information please click on the following link:
https://www.rootsanalysis.com/reports/view_document/medical-device-cros-market/226.html
The report features an extensive study of the current market landscape and future opportunities of contract research service providers focused on medical devices. The study also features an in-depth analysis, highlighting the capabilities of the various stakeholders engaged in this domain, across different regions of the globe. In addition to other elements, the study includes:
A detailed review of the overall landscape of medical device CROs.
An elaborate discussion on the various guidelines established by major regulatory bodies for medical device approval across North America, Europe, and Asia-Pacific and rest of the world.
Elaborate profiles of key players that specialize in offering services for both clinical and preclinical stage development of medical devices.
An analysis highlighting the key performance indicators used by sponsor companies to evaluate service providers engaged in this domain
A competitive benchmarking, highlighting the key focus areas of small, mid-sized and large companies, comparing their existing capabilities within and beyond their respective peer groups.
A detailed brand positioning analysis of leading industry players (shortlisted on the basis of strength of service portfolio).
A detailed geographical clinical trial analysis of ongoing and planned studies related to medical devices.
A detailed analysis of the mergers and acquisitions that have taken place in this domain during the period 2015-2020.
A survey analysis featuring inputs solicited from various experts who are directly / indirectly involved in providing contract research services to medical device developers.
A discussion on affiliated trends, key drivers and challenges, under a SWOT framework, which are likely to impact the industry’s evolution.
An elaborate discussion on the future opportunities / trends for the medical device outsourcing market that are likely to influence the growth of this domain over the coming years.
A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
Phase of Development
Clinical
Preclinical
Types of Preclinical Services Offered
Biocompatibility testing
Sterility and microbiology testing
Material characterization and analytical services
Others
Types of Clinical Services Offered
Clinical trial management
Data management
Regulatory affairs management
Consulting
Others
Device Class
Class I medical devices
Class II medical devices
Class III medical devices
Target Therapeutic Area
CNS disorders
Cardiovascular disorders
Oncological disorders
Bone disorders
Respiratory disorders
Pain management disorders
Ophthalmic disorders
Psychological disorders
Metabolic disorders
Others
Key Geographical Regions
North America
Europe
Asia-Pacific
Rest of the World
For more information please click on the following link:
https://www.rootsanalysis.com/reports/view_document/medical-device-cros-market/226.html
Transcripts of interviews held with the following senior level representatives of stakeholder companies
Lajos Sarosi (Chief Executive Officer and Co-founder, HungaroTrial)
Christopher Rupp (Vice President of Global Marketing and Commercial Operations, NAMSA)
Christian Wolflehner (General Manager, CW Research & Management)
Troy Mccall (Chief Operating Officer, CROMSOURCE)
Nazish Urooj (Senior manager, Medical & Clinical Operations, Metrics Research)
C. Omprakash (Technical Director and Partner, Vyomus Consulting)
Tania Persson (Director of Business Development, A+ Science)
Alexa Foltin-Mertgen (Business Development Manager, AtoZ-CRO)
Key companies covered in the report
Avania (formerly known as Factory CRO)
Charles River Laboratories
Clinlogix
CROMSOURCE
CSSi LifeSciences™
Eurofins Medical Device Testing
genae
IMARC Research
IQVIA
Medpace
NAMSA
Qserve Group
Regulatory and Clinical Research Institute (now a part of Covance)
WuXi AppTec
For more information please click on the following link:
https://www.rootsanalysis.com/reports/view_document/medical-device-cros-market/226.html
Other Recent Offerings
1. Contract Regulatory Affairs Management Market for Medical Devices, 2019-2030
2. Medical Device Labels Manufacturing Market, 2019 - 2030
3. Medical Device Contract Manufacturing Market, 2019-2030
Contact Information
Roots Analysis Private Limited
Gaurav Chaudhary
+1 (415) 800 3415
gaurav.chaudhary@rootsanalysis.com
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact:
Gaurav Chaudhary
gaurav.chaudhary@rootsanalysis.com
Roots Analysis
A430, 4th Floor,
Bestech Business Towers, Sector 66, Mohali, India
sales@rootsanalysis.com
+1 (415) 800 3415
+44 (122) 391 1091
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The medical device CRO market is estimated to be worth USD 15.7 billion in 2030, predicts Roots Analysis here
News-ID: 2189048 • Views: …
More Releases from Roots Analysis
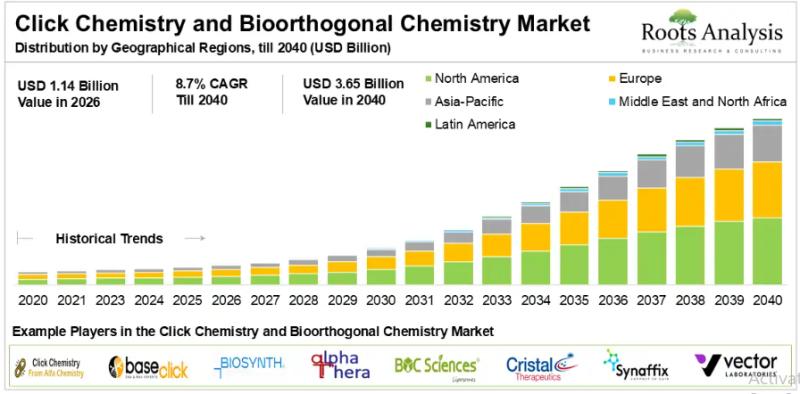
CLICK CHEMISTRY AND BIOORTHOGONAL CHEMISTRY: REVOLUTIONIZING DRUG DELIVERY
Click Chemistry was first coined by Karl Barry Sharpless at the 217th ACS National Meeting in 1999.1 It refers to a chemical synthesis approach wherein molecular building blocks combine efficiently and selectively, generating no or fewer by-products that can be easily purified without chromatographic separation techniques. Notably, there are various types of click chemistry reactions; amongst these, bioorthogonal chemistry is most prominently employed in biological systems without altering the molecule…
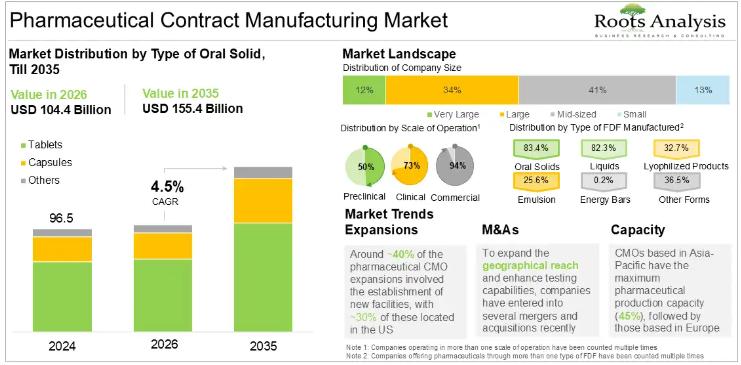
Pharmaceutical Contract Manufacturing Market CAGR To Reach 4.5% between 2025 and …
According to our latest market report "Pharmaceutical Contract Manufacturing Market by Type of Product Manufactured, Type of API, API Potency, Type of FDF, Dosage Form, Type of Oral Solid, Type of Packaging Offered, Scale of Operation, End User, Geographical Regions and Key Players: Industry Trends and Global Forecasts, till 2035", the pharmaceutical contract manufacturing market is estimated to be USD 100.3 billion in 2025. It is expected to reach USD…
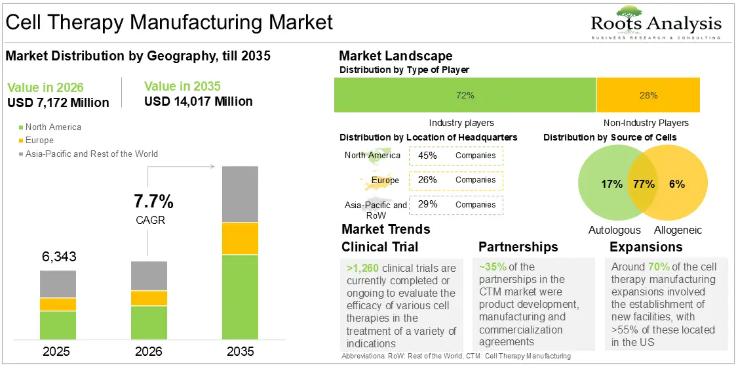
Cell Therapy Manufacturing Market CAGR To Exceed 8.25% by 2035, Due to the Growi …
According to our latest market report "Cell Therapy Manufacturing Market by Type of Cell Therapy, Source of Cells, Scale of Operation, Type of Manufacturer and Key Geographical Regions: Industry Trends and Global Forecasts, 2023-2035", the global cell therapy manufacturing market size is projected to reach USD 14,017 million by 2035 from USD 6,343 million in 2025, growing at a CAGR of 8.25% in the forecast period 2025-2035.
To request quote…
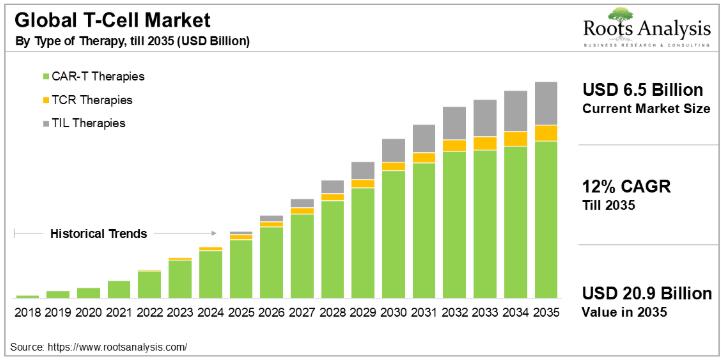
T-Cell Therapy Market Size to Hit USD 20.9 billion by 2035| Exclusive Report by …
Cancer is one of the leading causes of mortality across the world. As per the International Agency for Research on Cancer (IARC), by 2040, there are likely to be 27.5 million new cases and 16.3 million deaths related to cancer, annually. Although cancer therapeutics continue to be one of the most active areas, in terms of drug development, there is still a significant unmet need in this domain. In fact,…
More Releases for CRO
Contract Research Organization (CRO) Research: China CRO market size is projecte …
QY Research Inc. (Global Market Report Research Publisher) announces the release of 2025 latest report "Contract Research Organization (CRO)- Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031". Based on current situation and impact historical analysis (2020-2024) and forecast calculations (2025-2031), this report provides a comprehensive analysis of the global Wire Drawing Dies market, including market size, share, demand, industry development status, and forecasts for the next few…
Global Veterinary CRO & CDMO Market Size & Trends
According to a new market research report published by Global Market Estimates, the global veterinary CRO & CDMO market is expected to grow at a CAGR of 9.1% from 2023 to 2028.
The growing awareness regarding the importance of animal health and welfare has led to increased investments in veterinary pharmaceutical research and development. This awareness is driven by the rising concern for the well-being of pets, livestock, and animals used…
Global Contract Research Organization (CRO) Services Market
According to a new market research report published by Global Market Estimates, the Global Contract Research Organization (CRO) Services Market is expected to grow at a CAGR of 10.2% from 2023 to 2028.
The growth of the contract research organization (CRO) Services Market is fuelled by factors including the rising preference for outsourcing clinical trials, increasing investments in research and development by pharmaceutical and biopharmaceutical companies, the growing complexities in drug…
Contract Research Organization (CRO) Services Market - Efficiency, Expertise, Ex …
Newark, New Castle, USA: The "Contract Research Organization (CRO) Services Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Contract Research Organization (CRO) Services Market: https://www.growthplusreports.com/report/contract-research-organization-cro-services-market/7630
This latest report…
Biopharmaceutical CMO and CRO Market
The Biopharmaceutical CMO and CRO Market crossed US$ 32.25 billion in 2022 and is expected to hit US$ 54.13 billion by 2030, recording a CAGR of 6.5% during the forecast period
Pharmaceutical and biotech companies focus on R&D activities to innovate new molecules and therapeutic platforms to deal with chronic diseases and rare ailments; they also extensively invest in R&D activities to develop various therapeutic applications with strong medical and commercial…
Pracedo Promote Ellie Copp To CRO
Pracedo, a Mashfrog Group company that provides Salesforce consultancy and digital transformation to charities, NGOs, and enterprise customers, promotes Ellie Copp to Chief Revenue Officer.
Pracedo announced Ellie Copp as its new Chief Revenue Officer (CRO). Ellie joined Pracedo in 2019 and most recently held the position of Sales and Marketing Director. As CRO, she will continue to lead the commercial teams by overseeing all revenue-generating activities as Pracedo continues…
