Press release
Regulatory Information Management Sales Influenced by Improved Technological Arrangement during 2019 - 2029 | Key Players - DXC Technology , IQVIA Holdings , Veeva Systems , PAREXEL International , Lorenz Life Sciences , Aris Global LLC , AMPLEXOR , Spart
The regulatory information management (RIM) market is envisaged to surpass revenues worth US$ 893 Mn in 2019, according to a recent study by Fact.MR. Growing requirement for seamless regulatory submission data and product labeling management across enterprises is providing a fillip to the installation of regulatory information management software. Dire need of companies to alleviate the ‘time to market’ (TTM) cycle is a significant factor providing an impetus to the growth of regulatory information management market.Request a sample of this premium report @ https://www.factmr.com/connectus/sample?flag=S&rep_id=3741
The study opines that as the regional and international authorities continue to set the ever-changing reporting requirements, the call for proper RIM solution deployment will grow louder. The demand for regulatory information management solutions will be further assisted by the growing number of firms realizing the significance of the strategic role of product data.
According to the report, deployment of regulatory information management software for health authority management will remain high across pharmaceuticals industry, with the global deployment across different verticals estimated to surpass US$ 252 Mn in 2019. Reduction in manual errors in regulatory processes, along with an easy access to dossier, continues to contribute to the growth of the regulatory information management market.
A wide range of small and medium enterprises are implementing a broad regulatory information management platform to eliminate various data hand-offs and for better overall data quality and visibility. As per the study, global deployment of regulatory information management across small and medium enterprises exceeded US$ 513 Mn in 2018, and will witness a robust yearly growth in 2019 and ahead.
The study also highlights that the deployment of regulatory information management software is growing at a steady pace, driven by a plethora of categories. However, health authority management will particularly remain an attractive category. Furthermore, the demand for unified regulatory information management software will remain sustained in health authority management, as it enables effective control of data for a leaner, higher quality submission. This further translates to a more efficient review process for authorities and builds credibility, potentially reducing regulatory burden of enterprises, and thereby augmenting the deployment of unified regulatory information management platforms.
Browse full report along with TOC and List of Figures at: https://www.factmr.com/report/3741/regulatory-information-management-market
Evolving Regulatory Framework Triggering NPDs
The study opines that compliance modernization is no longer optional, with international authorities continuously setting new and often arduous reporting requirements. In view of the constantly changing regulatory paradigms, enterprises with different verticals, such as pharmaceuticals, medical devices, and nutraceuticals are expecting more from the software that helps them log and keep track of everything. This has further accelerated the trend of new product development (NPD) in the regulatory information management market, finds the study. Additionally, sensing the pain points related to disparate systems, exacerbated by IDMP (Identification of Medicinal Products) requirements, more players in regulatory information management market are drifting to unified platforms through NPD.
As per the study, growing demand for a solution that can manage, control and fasten the process of bringing novel products to market, maintain existing products, and manage interactions with authorities is favoring deployment of RIM software. Furthermore, high penetration of small scale companies in regulatory information management market is another significant factor that continues to shape the growth of this industry.
As per the Fact.MR study, North America will continue to lead the market for regulatory information management due to growing stringency of rapidly changing norms enforced by several regulatory bodies in the region. The study opines that services segment of regulatory information management will underpin higher revenues in North America owing to high quality assurance and support benefits offered by regulatory management organizations in this region.
Although developed economies are spearheading the regulatory information management market, developing regions are estimated to pick pace through 2029. The study opines that East Asia is envisaged to remain the high growth market for regulatory information management, as verticals of regional companies have plans afoot for significant investments in platforms for proper regulatory information management. A plethora of companies across the emerging economies of East Asia, China in particular are transitioning toward regulatory information management software to alleviate their product time to market, amid growing clinical trials in the region.
Ask to our Industry Expert @ https://www.factmr.com/connectus/sample?flag=AE&rep_id=3741
Contact Us
Fact.MR
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Email: sales@factmr.com
Web: https://www.factmr.com/
Blog: https://factmrblog.com/
Read Industry News at - https://www.industrynewsanalysis.com/
About Fact.MR
Fact.MR is a fast-growing market research firm that offers the most comprehensive suite of syndicated and customized market research reports. We believe transformative intelligence can educate and inspire businesses to make smarter decisions. We know the limitations of the one-size-fits-all approach; that's why we publish multi-industry global, regional, and country-specific research reports.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Information Management Sales Influenced by Improved Technological Arrangement during 2019 - 2029 | Key Players - DXC Technology , IQVIA Holdings , Veeva Systems , PAREXEL International , Lorenz Life Sciences , Aris Global LLC , AMPLEXOR , Spart here
News-ID: 1801053 • Views: …
More Releases from Fact.MR
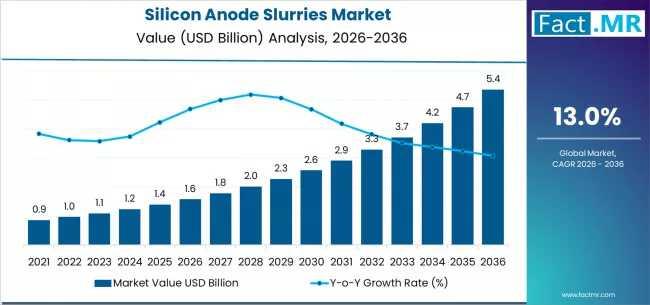
Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…

Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…
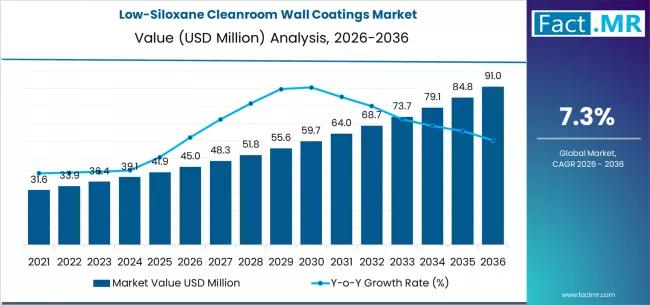
Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…

Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…
