Press release
Dynamic Movement of Clinical Trial Management Systems Market through 2018-2028 | Key Players are Bioclinica, Oracle Corporation, DSG Inc., Forte Research Systems Inc., Veeva Systems, etc.
In order to study the various trends and patterns prevailing in the concerned market, FactMR has included the latest Forecast report on clinical trial management systems market to its vast database. This study offers data about the prime regions operating in the clinical trial management systems market, along with their production, consumption, revenue and market share details. Further, the intelligent report also anticipates that the market would grow at a constructive CAGR until 2028. Clinical trial management systems are central to eClinical environments, and considering the paramount focus on product safety and efficiency, there is a palpable shift towards better functionality and standardization.Request a Sample Report Here - https://www.factmr.com/connectus/sample?flag=RC&rep_id=832
The evolutions in how clinical trials are conducted has meant that vendors are hard pressed to offer advanced solutions in addition to core functionalities such as site management, monitoring, and tracking. The need for innovation in clinical trials is also driven by mandates from regulatory authorities. Emphasis on transparency throughout the study process and adoption of risk-based approaches to clinical trial design influence the clinical trial management systems market.
The growing use of ‘Software as a Service (SaaS)’ system that enables web-based access to trial management systems signifies an undergoing shift in the clinical research industry. Web-based clinical trial management systems are becoming pervasive, and are expanding into new areas of clinical research. Although adopting web-based clinical trial management systems come with their own share of challenges, their benefits often outweigh the challenges.
The penetration of web-based clinical trial management systems has grown manifold in the last five years or so. Access to information on a real-time basis, coupled with the feasibility of analyzing results quickly are among the leading factors that have fuelled adoption. The virtual storage option has given pharmaceutical and biotechnology companies the flexibility in terms of cost and computing power. It is no surprise then that web-based clinical trial management systems account for nearly 70% revenue share of the market. The preference for web-based clinical trial management systems is likely to continue during the assessment period as well. During the course of the review period, web-based clinical trial management systems will consistently hold nearly 70% revenue share.
Browse Full Report on Global Market with TOC - https://www.factmr.com/report/832/clinical-trial-management-systems-market
The transition to web-based clinical trial management also gives end-users the tools to centralize computational and research tools. The flexibility, affordability, and advanced technology make web-based clinical trial management the preferred deployment model for end-users, however, challenges persist.
End-users have often a misplaced, exaggerated notion about the security apparatus of clinical trial management systems market. Although vendors have made investments in improving the security apparatus of web-based cloud security models, a concerted effort from all stakeholders is necessary if web-based clinical trial systems are to witness further penetration. Another key factor for clinical trial management systems to find the right vendors that is sensitive to the particular elements of life science and clinical trial data security and regulations. Of particular importance is compliance with FDA’s Title 21 Code of Federal Regulations. Regulatory compliance also entails standardization in approval from health services and insurance companies.
Considering the slew of advantages clinical trial management systems have over traditional systems, the future belongs to web-based clinical trial management systems. The transition in the clinical trial management systems has been undergoing for some time now, and web-based procedures now account for a majority share of the market. However, it is also pertinent to mention that there is still a level of skepticism and reluctance in the clinical trial industry, especially in terms of data security and privacy. Availability of platforms and cloud-based products that utilize big data technology to optimize the cost of clinical development process can reduce some level of uncertainty.
To Buy Market Report, Check - https://www.factmr.com/checkout/501/S
About FactMr
FactMR is a fast-growing market research firm that offers the most comprehensive suite of syndicated and customized market research reports. We believe transformative intelligence can educate and inspire businesses to make smarter decisions. We know the limitations of the one-size-fits-all approach; that's why we publish multi-industry global, regional, and country-specific research reports.
Contact Us
FactMR
11140 Rockville Pike
Suite 400, Rockville, MD 20852
United States
Email: sales@factmr.com
Web: https://www.factmr.com/
Blog: https://factmrblog.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Dynamic Movement of Clinical Trial Management Systems Market through 2018-2028 | Key Players are Bioclinica, Oracle Corporation, DSG Inc., Forte Research Systems Inc., Veeva Systems, etc. here
News-ID: 1638637 • Views: …
More Releases from Fact.MR

2036 Global Freeze-dried Pea Isolates Market Intelligence Report: Technology Shi …
The global freeze-dried pea isolates market is projected to witness robust expansion through the next decade as food manufacturers and consumers increasingly seek high-quality, plant-based protein ingredients that support clean-label products and sustainable nutrition. In 2025, the freeze-dried pea isolates market is valued at USD 1.1 billion, and it is expected to reach USD 2.8 billion by 2035, representing an absolute increase of USD 1.7 billion over the forecast period.…
Executive Report: Future of the Global High-concentrated Protein Market - Key Dr …
The global high-concentrated protein market is anticipated to record significant expansion over the next decade as consumer awareness of protein's nutritional benefits rises across food & beverage, Food supplements, sports nutrition, clinical nutrition, and animal feed sectors. Driven by trends toward healthy lifestyles, balanced diets, and functional food formulations, demand for concentrated protein ingredients is expected to further increase as individuals and manufacturers alike prioritise high-quality protein sources.
Industry analyses indicate…
Hydrogen Vehicles Market Forecast 2026-2036: Market Size, Share, Competitive Lan …
The global hydrogen vehicles market is projected to experience substantial growth over the next decade as manufacturers, governments, and consumers increase investments in zero-emission transportation technologies. In 2025, the hydrogen vehicles market is valued at USD 12.5 billion, and it is expected to reach USD 47.8 billion by 2035, reflecting an absolute increase of USD 35.3 billion over the forecast period. This expansion corresponds to a compound annual growth rate…

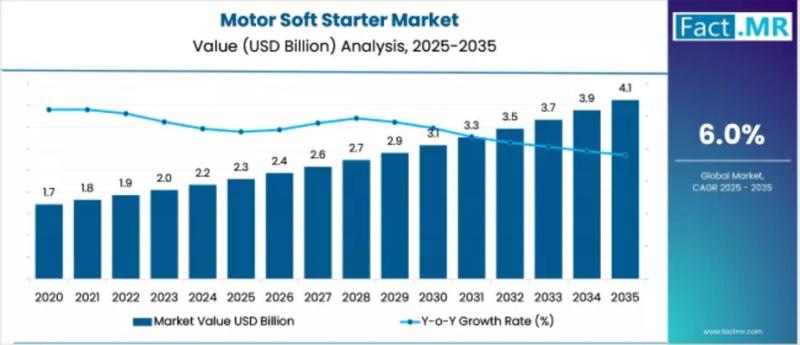
Motor Soft Starter Market Outlook 2026-2036: Strategic Trends, Innovation Driver …
The motor soft starter market is poised for significant growth over the next decade as industries seek to improve electric motor performance, reduce mechanical stress, and enhance energy efficiency in industrial operations. In 2025, the motor soft starter market is valued at USD 2.8 billion, and it is projected to reach USD 6.9 billion by 2035, representing an absolute increase of USD 4.1 billion over the forecast period. This growth…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
