Press release
The growing prevalence of cancer and increased FDA approvals are the prime factors expected to drive the growth of the immune checkpoint inhibitors market
Attributed to growing demand for the development of biologics targeting cancer therapy, PMR predicts that the immune checkpoint inhibitors will present lucrative opportunities to investors in the near future – as quoted by a research expert at Persistence Market Research (Healthcare & Life Sciences).To know key findings Request Sample Report @: https://www.persistencemarketresearch.com/samples/20122
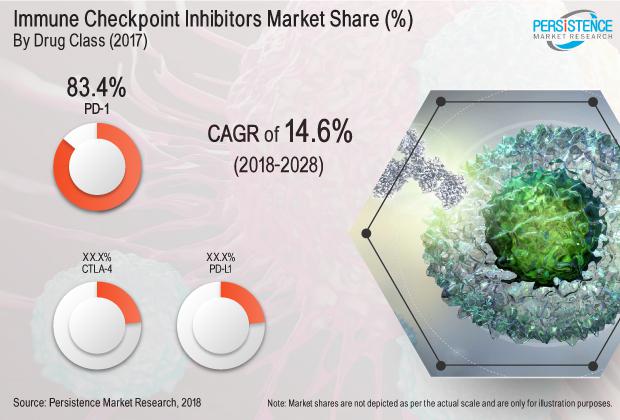
Expanding at a booming CAGR of 14.6%, the global market for immune checkpoint inhibitors market is expected to reach a value beyond US$ 35 Bn over 2018-2026. Growing prevalence of various types of cancers and expanding healthcare expenditure will remain the most prominent drivers of immune checkpoint inhibitors market over the forecast period. Besides cancer prevalence, increasing FDA approvals and growing use of immune-oncology products in treating cancers will also push the demand for immune checkpoint inhibitors in the near future. However, high price point associated with both drug development and treatment will continue to restrict adoption. Frequently observed last stage failure will also remain a major concern in the long run.
“Our extensive company share analysis predicts that the top two players in the global immune checkpoint inhibitors market currently hold a massive revenue share of over 95%. Based on the exhaustive assessment of each drug class in the global immune checkpoint inhibitors market, we arrived at the forecast that the PD-1 will continue dominance in the market, primarily attributed to rapid FDA approvals to PD-1 products,” explained an expert senior research analyst working with the healthcare and life sciences domain at Persistence Market Research.
Request for Report Methodology @: https://www.persistencemarketresearch.com/methodology/20122
FDA Approvals Continue to Encourage North American Market for Immune Checkpoint Inhibitors
North America is likely to continue monopoly in the global market for immune checkpoint inhibitors with over 65% share of the total market revenue. Large number of FDA approvals, expanding application base in treatments for various cancer types, and increasing combination drug approvals will play a pivotal role in shaping the market for immune checkpoint inhibitors in North America. According to PMR’s regional analysis of global immune checkpoint inhibitors market, Asia Pacific will demonstrate vigorous growth throughout the projection period – at the double digit CAGR of over 17%.
High Potential Combination Therapies to Open New Doors of Opportunities
With a strong reference post success of the promising results delivered by Bristol-Myers Squibb using a combination of Yervoy and Opdivo, the market for immune checkpoint inhibitors has been witnessing several more combination therapy products in the pipeline. Such a scenario is responsible for the current situation of the immune checkpoint inhibitors marketplace that reflects a major paradigm shift of companies from conventional mono-therapies to combination therapies for better end results. While a considerable number of combination therapies introduced by various companies have been successfully contributing to the efforts in transforming oncology, the global market for immune checkpoint expects successful introduction of more such therapies. One of those in the pipeline includes the combination with target specific mAbs, chemotherapy, and other checkpoint inhibitors.
Key Players to Invest Efforts in Developing Products to Traverse Diverse Indications
The global immune checkpoint inhibitors market is competitive yet consolidated due to strong presence of established players; however, a few prominent brands such as Tecentriq have strategically maintained a consistent position in the market since their first product launch. A majority of leading players participating in the global immune checkpoint inhibitors marketplace are concentrating on incorporating value addition programs for regulating the customer base through enhancement of product offerings to suit a diverse range of indications.
Get access to full summary @: https://www.persistencemarketresearch.com/market-research/immune-checkpoint-inhibitors-market.asp
Established companies in the global immune checkpoint inhibitors market landscape are striving to enhance therapeutic applications of their existing product offerings. Moreover, a number of key players are also eyeing the high potential markets emerging in developing countries such as KSA and South Africa by seeking FDA approvals to their products in these markets.
About Us
Persistence Market Research (PMR) is a third-platform research firm. Our research model is a unique collaboration of data analytics and market research methodology to help businesses achieve optimal performance.
To support companies in overcoming complex business challenges, we follow a multi-disciplinary approach. At PMR, we unite various data streams from multi-dimensional sources. By deploying real-time data collection, big data, and customer experience analytics, we deliver business intelligence for organizations of all sizes.
Persistence Market Research
305 Broadway
7th Floor, New York City,
NY 10007, United States,
USA – Canada Toll Free: 800-961-0353
Email: sales@persistencemarketresearch.com
Web: http://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The growing prevalence of cancer and increased FDA approvals are the prime factors expected to drive the growth of the immune checkpoint inhibitors market here
News-ID: 1521985 • Views: …
More Releases from Persistence Market Research

Aseptic Packaging Market Set to Grow to US$136.5 Bn by 2033 Driven by Rising Foo …
Introduction: Aseptic Packaging Market Overview and Industry Evolution
The aseptic packaging market has emerged as a cornerstone of modern food, beverage, and pharmaceutical preservation systems. As global consumers increasingly seek safe, long-lasting, and convenient packaged products, aseptic packaging solutions have gained substantial importance. This technology allows products to be sterilized separately from packaging materials and filled in a sterile environment, ensuring extended shelf life without refrigeration or preservatives. The rising demand…
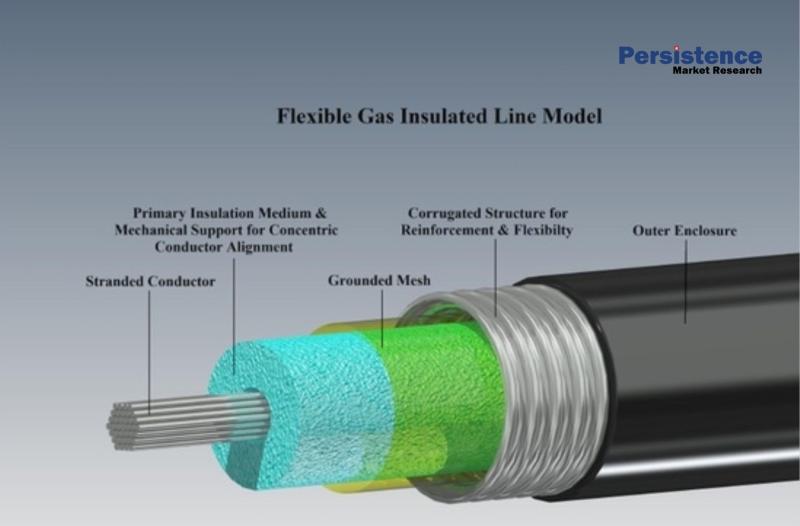
Gas-Insulated Transmission Line (GIL) Market Expected to See Growth to US$836.9 …
Introduction: Rising Demand for Advanced Power Transmission Solutions
The global energy landscape is undergoing a major transformation as nations accelerate investments in renewable energy, grid expansion, and reliable electricity distribution networks. With rapid urbanization, industrialization, and digital infrastructure growth, the demand for uninterrupted and efficient power transmission has never been higher. Traditional overhead transmission systems, while widely used, face challenges related to land constraints, environmental exposure, and transmission losses. This has…
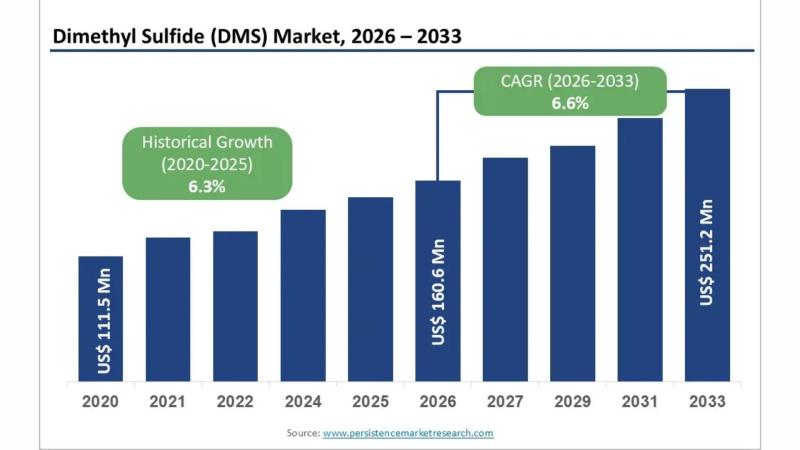
Dimethyl Sulfide (DMS) Market Anticipated to Reach US$ 251.2 Mn by 2033, Driven …
Market Overview: Expanding Demand for Dimethyl Sulfide Across Industries
The Dimethyl Sulfide (DMS) market is witnessing consistent growth due to its widespread applications across food processing, pharmaceuticals, agriculture, and chemical manufacturing. Dimethyl sulfide is an organosulfur compound known for its distinctive odor and reactive properties, making it a crucial intermediate in multiple industrial processes. In food and beverage applications, it is valued for flavor enhancement, while in chemical manufacturing, it serves…
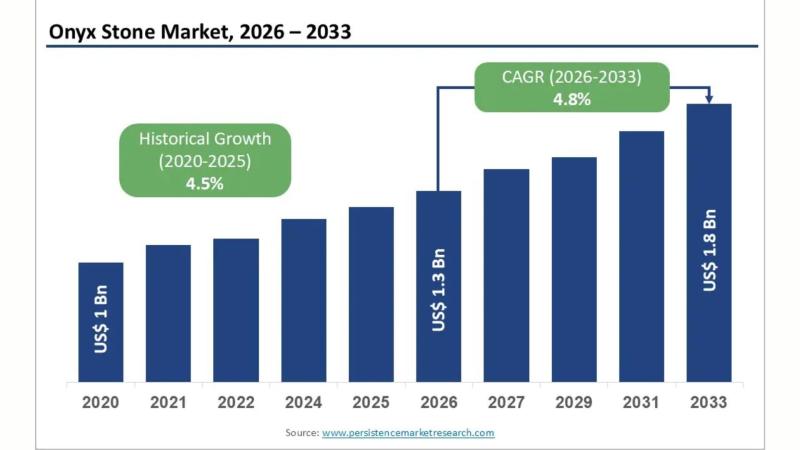
Onyx Stone Market to Surge to Reach US$ 1.8 Bn by 2033 Driven by Construction & …
Market Overview: Rising Demand for Premium Natural Stones
The onyx stone market has gained remarkable prominence in recent years, driven by increasing demand for luxury natural stones in architecture, interior décor, and artistic applications. Onyx, known for its translucent appearance and unique veining patterns, has become a preferred choice for premium residential and commercial projects. The material's ability to elevate aesthetic appeal while maintaining durability has attracted designers, architects, and homeowners…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…