Press release
Global Clinical Trial Management Systems Market: Key Facts and Forecast Predictions Presented Until 2028 || Top Market Competitors- Medidata Solutions, PAREXEL International, International Business Machines Corporation, Bioclinica, Oracle Corporation, Cog
Web-based clinical trial management systems continue to be the preferred mode of deployment for pharmaceutical and biotechnology companies, finds a new study by Fact.MR. The report projects that web-based clinical trial management systems will remain pervasive in the clinical trial management systems market during the review period 2018-2028, gaining an impressive US$ 116 Mn in incremental opportunity in a decade.Know the methodology behind the report - https://www.factmr.com/connectus/sample?flag=RM&rep_id=832
Clinical trial management systems are central to eClinical environments, and considering the paramount focus on product safety and efficiency, there is a palpable shift towards better functionality and standardization. The clinical trial management systems market continues to be influenced by a range of factors – ranging from macroeconomic to industry-specific. On the back of a multi-pronged factors, the clinical trial management systems market is likely to tread along steadily during the assessment period.
Clinical Trial Management Systems Market to Surpass US$ 1 Billion During the Course of Forecast Period
The report is bullish on the prospects of clinical trial management systems market, and expects revenues to surpass US$ 1 billion during the course of the forecast period. Growth will be induced by a range of macroeconomic and industry-specific factors, with growing awareness on the critical role of clinical trial management systems in improving workflow influencing investment.
Clinical Trial Management Systems Market: Sensitization on Benefits over Paper-based Driving Growth
The report finds that sensitization about the operational benefits of clinical trial management system over manual paper-based process has been a key factor for increased adoption. While a majority of end-users are using clinical trial management systems in-house, a significant percentage of end-users also outsource to vendors, such as CROs and SMOs. Outsourcing of site monitoring, site management, and schedule tracking functions was highest among end-users.
According to the report, standardization, SaaS-based solutions, and flexibility in terms of pricing and licensing are among the key evolutions in the clinical trial management system market. The increasing complexity in clinical trials has meant that pharmaceutical companies prefer outsourcing clinical trial execution to contract organizations. Outsourcing is likely to gain further momentum during the assessment period as proliferation of specialty clinical research organizations and academic research organizations (AROs) witnesses a spike.
View Full Report with TOC @ https://www.factmr.com/report/832/clinical-trial-management-systems-market
The report finds that revenues generated from clinical trial management software are higher than services, and the status quo is unlikely to remain unchanged during the assessment period. The revenues generated from clinical trial management software is nearly 3x of services in 2018, and both these segments are likely to grow at similar CAGR during the assessment period.
Preference for enterprise-based clinical trial management systems is higher among end-users. The preference for enterprise-based clinical trial management systems can be gauged by the fact that this segment accounted for nearly three-fourth revenue share of the market in 2018. The preference for enterprise-based clinical trials is likely to continue unabated during the review period.
Adoption of clinical trial management systems is mainly concentrated among pharmaceutical companies, contract research organizations (CROs), and healthcare providers. Pharmaceutical companies account for nearly three-fourth revenue share of the clinical trial management systems market, with healthcare providers accounting for a miniscule revenue share.
The penetration rate of clinical trial management systems is higher in the US and EU5, with pharmaceutical companies and other end-users adopting a proactive approach towards adoption of advanced software and services. The proliferation of clinical trial management systems in the US is reflected by the statistic that the country accounts for nearly 40% revenue share of the global clinical trial management systems market. The adoption rate is also impressive in Canada, albeit the market is likely to grow from a small base.
According to the report, adoption of clinical trial management systems in Europe is concentrated in Germany, U.K., Spain, Italy, and France. These five markets collectively account for nearly 68% revenue share of the clinical trial management systems market. Germany is likely to retain its position as the most lucrative market for clinical trial management systems, whereas growth is likely to be sluggish in the U.K.
Table of Content:
Chapter 1 Executive Summary
Chapter 2 Global CTMS Market Overview
2.1 Introduction
2.2 Market Definition
2.3 Scope of the report
Chapter 3 Market Dynamics
3.1 Key Growth Drivers
3.1.1 The Role of CTMS in Improving Workflow
3.1.2 Importance in the eClinical Framework
3.1.3 Growing Importance of CTMS in eClinical Value Chain
3.1.4 Streamlining Research Activities
3.2 Key Market Restraints
3.2.1 Evolving Roadblocks
3.2.2 Complex Trial Management
3.3 Opportunities
3.3.1 Progress in Web Technology
3.3.2 Increasing Biomedical Research Funding
3.3.3 Key Trends
3.3.4 Increasing Outsourcing Activities
Continued……………..
Clinical Trial Management Systems Market Report is available for Purchase @ https://www.factmr.com/checkout/818/S
Future of Web-Based Clinical Trial Management Systems Market
Considering the slew of advantages clinical trial management systems have over traditional systems, the future belongs to web-based clinical trial management systems. The transition in the clinical trial management systems has been undergoing for some time now, and web-based procedures now account for a majority share of the market. However, it is also pertinent to mention that there is still a level of skepticism and reluctance in the clinical trial industry, especially in terms of data security and privacy. Availability of platforms and cloud-based products that utilize big data technology to optimize the cost of clinical development process can reduce some level of uncertainty.
About FactMR
FactMR is a fast-growing market research firm that offers the most comprehensive suite of syndicated and customized market insights reports. We believe transformative intelligence can educate and inspire businesses to make smarter decisions. We know the limitations of the one-size-fits-all approach; that's why we publish multi-industry global, regional, and country-specific research reports.
Contact Us
FactMR
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Email: sales@factmr.com
Web: https://www.factmr.com/
Read Industry News at- https://www.industrynewsanalysis.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Clinical Trial Management Systems Market: Key Facts and Forecast Predictions Presented Until 2028 || Top Market Competitors- Medidata Solutions, PAREXEL International, International Business Machines Corporation, Bioclinica, Oracle Corporation, Cog here
News-ID: 1434942 • Views: …
More Releases from Fact.MR
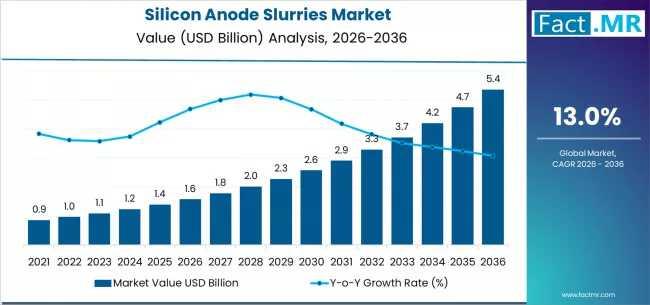
Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…

Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…
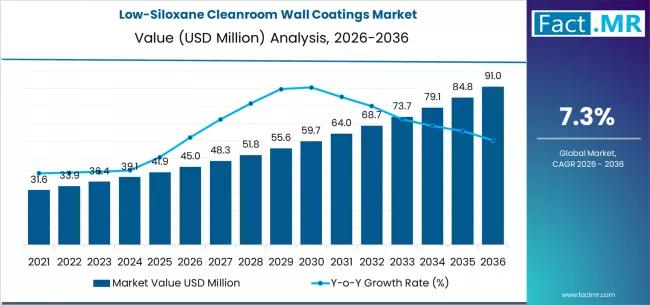
Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…

Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
