Press release
SpinDiag closes second financing round of EUR 3.0 million (USD 3.4 million) to complete product development
Freiburg im Breisgau, Germany / November 2018 – One year after its seed financing, SpinDiag GmbH closes a second financing round of EUR 3 million (USD 3.4 million) as planned. The financing will enable SpinDiag to complete the product development of its first product for screening for antibiotic-resistant bacteria and to start clinical trials. This will pave the way for regulatory approval in the EU.To finance the current Series A, SpinDiag won over Mr. Werner Geissler as well as its existing investors once again. “Mr. Geissler is a global sales and marketing expert who can provide us with excellent support in making the best possible market entry in the EU and the US”, Dr. Daniel Mark, co-founder and CEO of SpinDiag, points out. “From the very beginning, I was convinced by the market fit and the scalability of SpinDiag’s products as a solution for urgent problems of global scale” reports Mr. Geissler. In his over 30 years with the Procter & Gamble Company (P&G), Mr. Geissler most recently was Vice Chairman of Global Operations with worldwide responsibility for approximately $80 billion in revenue and over 100,000 employees. Prior, he took care of important P&G brands such as Ariel, Gillette, Max Factor, Oral-B from various locations in Germany, Japan, Turkey, Switzerland and the US.
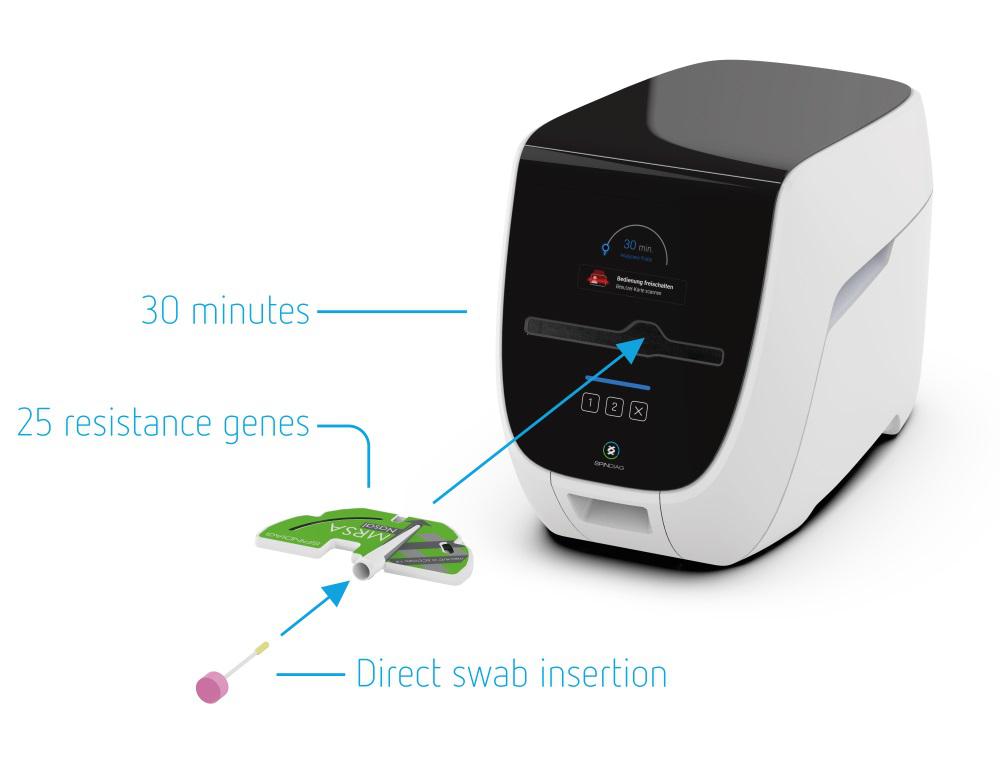
SpinDiag's fast and reliable diagnostic system will enable easy and efficient infection diagnostics. For the first time, healthcare facilities will be able to economically test all at-risk patients for all relevant antibiotic-resistant bacteria at their admission with SpinDiag’s first product, immediately decide on hygiene measures and may thus treat carriers of such bacteria in isolation from other patients. This will enable an efficient and safe admission process to increase patient safety and avoid costs for unnecessary isolation or even outbreaks.
Antibiotic-resistant bacteria detected by the test, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), carbapenemase-producing (CPO/CRE) and extended-spectrum betalactamase-producing (ESBL) organisms, were recently attributed responsibility for more than 30,000 deaths per year in the EU in a study published by the European Centre for Disease Prevention and Control (ECDC). These figures have more than doubled since 2007. In addition, 75% of them were hospital-acquired infections, so-called nosocomial infections. This makes prevention particularly important. “The SpinDiag system will provide healthcare facilities with a standardized, efficient and economical screening process that transparently focuses on patient safety,” emphasizes Dr. Mark Keller, co-founder and Chief Product Officer of SpinDiag.
“It is important to us to make SpinDiag a sustainable, innovative and valuable investment in the future for everyone involved: Future products in the SpinDiag family include tests for the diagnosis of sexually transmitted infections (STI), respiratory tract infections (RTI), and resistant tuberculosis (TB),” emphasizes Dr. Oliver Strohmeier, co-founder and CTO of SpinDiag. He adds: “With the product development status achieved to date, SpinDiag has already been able to eliminate all significant technical risks from the project for its first and subsequent products.” All components were successfully integrated into the system and pilot trials at the University Hospital of Freiburg were completed with very good results. At the same time, SpinDiag was able to underpin its unique selling proposition of an outstandingly intuitive and simple operation of the system by successful usability tests with health care professionals. “Usability is the key to ensure the lowest possible workload and the safest and quickest possible test execution,” said Keller. In April this year, at the 28th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Madrid (Spain), SpinDiag presented its system to the general public for the first time, which was very well received by the wide range of professionals. The event resulted in valuable contacts to established corporations as potential strategic partners, as well as to sales partners and future pilot customers. In addition to this preparation of the EU market, SpinDiag successfully carried out the planning for FDA approval for the US market with the German Accelerator Life Sciences (GALS) and deepened its understanding of the American market.
According to a report by Grand View Research, the global market for point-of-care diagnostics of infectious diseases is expected to reach USD 7.2 billion by 2020. SpinDiag will address this market with a widely patented device platform that will deliver reliable results in laboratory quality and with great efficiency – from integration into existing processes, to outstanding ease and safety of use, to extremely fast availability of results. In short: an economical system for increased patient safety.
About SpinDiag: SpinDiag GmbH was founded in 2016 and is based in Freiburg im Breisgau, Germany. Based on a widely patented centrifugal microfluidics platform, the company is developing a point-of-care diagnostic system as a safe, simple and efficient rapid test for numerous infectious diseases. The first product will enable the guideline-compliant screening of patients for antibiotic-resistant bacteria at their admission. The company has already received numerous awards, including multiple from experts in the healthcare sector such as BBraun in the code_n competition and the Techniker Krankenkasse together with Handelsblatt in the health-i competition.
SpinDiag GmbH, Dr. Daniel Mark
Engesserstr. 4a
79108 Freiburg im Breisgau
+49 761 203 73246
info@SpinDiag.de
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release SpinDiag closes second financing round of EUR 3.0 million (USD 3.4 million) to complete product development here
News-ID: 1413767 • Views: …
More Releases from SpinDiag GmbH

Spindiag seeks strategic investors for 15-min POC multiplex PCR system amid prel …
Spindiag GmbH invites strategic investors to join in on its innovation of a 15-minute point-of-care multiplex PCR system amid preliminary insolvency proceedings
Freiburg/Breisgau, Germany, July 10, 2023 - Spindiag GmbH, a start-up company in the field of molecular in-vitro diagnostics, has announced the opening of preliminary insolvency proceedings as a result of challenging market conditions caused by the rapid decline of COVID-19 testing. The company remains operational and is actively seeking…

Freiburg based Spindiag GmbH and Hahn-Schickard research institute receive six m …
The Economic Committee of Baden-Wuerttemberg's Ministry for Economic Affairs, Labour and Housing Construction approved of funding in fast track authorisation
Nested PCR test for results in about 35 minutes directly at the point of care
Freiburg im Breisgau, April 7, 2020 - Spindiag GmbH, based in Freiburg im Breisgau, is a medical technology start-up that arose out of the renowned Hahn-Schickard Institute for Microanalytical Systems four years ago. Together with Hahn-Schickard, the…
Spindiag’s continued road to success: Expansion of Series A with an additional …
- With new and current investors, the medtech startup in Freiburg takes the next big step in launching its innovative technology for the rapid detection of multidrug-resistant bacteria.
- The additional money will enable industrializing the production and securing CE-IVD approval for the EU market of an MRSA (methicillin-resistant Staphylococcus aureus) rapid test planned for launch in 2020.
- A recent study on the usability of Spindiag’s point-of-care system confirms its easy,…
SpinDiag Raises 1.6 Mio. € To Bring Point-of-Care Screening For Antibiotic Res …
The Freiburg-based startup SpinDiag GmbH recently closed a 1.6 Mio. EUR seed-round with three private investors. The team developed a revolutionary point-of-care screening system for testing patients for antibiotic-resistant bacteria at their admission to hospitals and almost instantly so. The seed-capital will make it feasible to bring SpinDiag’s system from its current laboratory environment to first tests in hospitals. “Testing in hospitals will not only be an important milestone for…