Press release
Pretreatment testing of SIMFO anticancer drug on patient blood
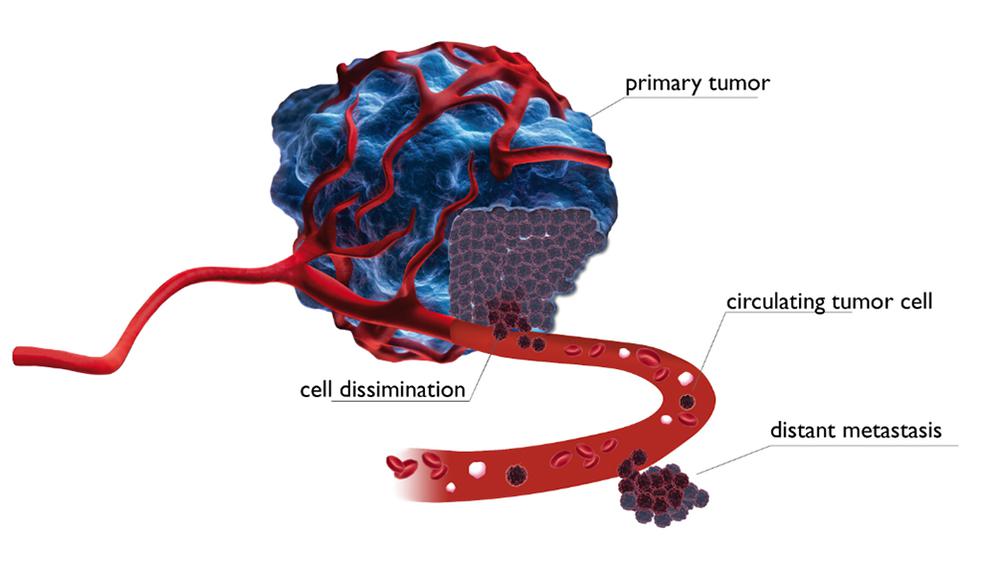
Dubai / Bayreuth - At the 2nd World Conference for Drug Discovery and Therapy, Prof. Dr. Katharina Pachmann (Jena University) presented a worldwide unique approach in oncology that can detect circulating tumor cells in the blood of cancer patients.After tumor removal, any remaining, washed out tumor cells are responsible for the development of metastases, which shorten survival time in most patients. A good, often metastasis-free prognosis of survival is achieved if the number of tumor cells present in the blood can be significantly reduced through chemotherapy, or in the case of breast cancer, with hormone therapy. Based on these findings, the impact of treatment on this remnant tumor burden in the blood can be easily determined.
With SIMFO, the efficacy of a drug intended for treatment on the tumor cells in the blood of each patient can already be tested in vitro prior to administration using the MAINTRAC method. A good correlation was seen between in vitro results and actual efficacy. In many cases, the results were decisive for the subsequent treatment.
With the MAINTRAC method it is possible for the first time to test the efficacy of a treatment prior to administering the drug. Currently, there is no other direct method available in the treatment of cancer, particularly in adjuvant (preventive) therapy, to check treatment response.
To cut down on the development time of new anti-cancer drugs, the MAINTRAC method may also be used prior to clinical trials. While the efficacy of the drug is already being studied in vitro with patient cells, MAINTRAC can assist at the same in the targeted use of animal testing and Phase I and Phase II studies.
SIMFO, Spezielle Immunologie Forschung + Entwicklung GmbH was founded in 1999 by Dr. med. Ulrich Pachmann. SIMFO GmbH is primarily a contract research institute for the development of new testing methods in the fields of hematology and oncology.
Key areas are quality assurance of platelet testing, validation of tests for treatment-dependent hemocytopenia and tests for drug sensitivity testing of tumor cells and the asservation of these cells from the peripheral blood.
SIMFO services include specialist literature searching as well as strategic laboratory development consultancy.
Further information:
Transfusion Center Bayreuth
Peter Pachmann
Company Communication
SIMFO Spezielle Immunologische Forschung + Entwicklung GmbH
Tel. 0049(0) 921 850 200
Fax 0049 (0)921 850 203
presse@simfo.de
www.simfo.de
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pretreatment testing of SIMFO anticancer drug on patient blood here
News-ID: 125579 • Views: …
More Releases from SIMFO Spezielle Immunologie Forschung + Entwicklung GmbH
SIMFO to present maintrac liquid biopsy at the BIO International Convention 2017
Maintrac Liquid Biopsy recognized by the European Commission as one of the most innovative procedures in circulating tumor cells
As one of the 15 most innovative European companies SIMFO GmbH was invited by the European Commission to the Bio International Convention (BIO) 2017 to present maintrac® liquid biopsy under the patronage of the EU. Small and medium-sized enterprises are given the opportunity to obtain comprehensive information about the US healthcare and…
maintrac® launches Immunotherapy PDL-1 blood test
Immunotherapy was one of the big topics at ASCO (American Society of Clinical Oncology) this year. Because of its potential, ASCO named it "The 2016 Clinical Cancer Advance of the Year". Immunotherapy is among today’s most promising approaches to improving the outcomes of patients.
Obtaining PD-L1 status previously required an invasive tissue biopsy. The highest quality liquid biopsy maintrac ® now makes an invasive procedure obsolete. A simple blood test, the…
More Releases for Pachmann
Ki67 as an additional marker on circulating tumor cells
Ki67 is a nuclear protein that is associated with cell proliferation. Ki67 is present during all active phases of the growth cycle, but is absent in resting (quiescent) cells. Ki67 is identified with the IHC method. The presence of Ki67 protein on circulating tumor cells indicates the percentage of circulating tumor cells in the active growth phase of the cell cycle. Higher amounts are associated with an increased risk of…
Antithrombotic management plan for cancer patients
In Germany Thrombosis occurs 470.000 timesevery year - as often as a new cancer is diagnosed. With 44.000 deadly lung artery embolisms every year, thrombosis is very dangerous.
Around 30 independent risk factors are known. One of the more important risk factors seems to be the amount of intracirculatory tissue factor molecules which is brought into circulation bound to the circulating epithelial cells.
Tissue factor is always active to start the…
maintrac® launches Immunotherapy PDL-1 blood test
Immunotherapy was one of the big topics at ASCO (American Society of Clinical Oncology) this year. Because of its potential, ASCO named it "The 2016 Clinical Cancer Advance of the Year". Immunotherapy is among today’s most promising approaches to improving the outcomes of patients.
Obtaining PD-L1 status previously required an invasive tissue biopsy. The highest quality liquid biopsy maintrac ® now makes an invasive procedure obsolete. A simple blood test, the…
