Press release
Authorized Generics Market Plying for Significant Growth During 2024
A generic drug is a duplicate copy of original branded drug, which has same dosage form, active pharma ingredients (API), strength, route of administration, and also same intended use as the branded one. Regulatory authorities and governments have mandated that an authorized generic drug has to medicinally correspond to the branded drug and sanctioned as an Abbreviated New Drug Application (ANDA) by the Food and Drug Administration (FDA). An authorized generic is the branded company’s individual product, but repackaged and marketed as generic drug either via subsidiary or third party. These are already approved as a New Drug Application by the FDA, only they are promoted via private label. The authorized generics market grew rapidly in the past few years as these provide consumers with branded quality drugs at generic prices. Presently, there is a growing trend of original maker giving approvals to a subsidiary or a private label distribution company to sell its brand name drug as a generic drug at a subsequently low price.Read Report Overview @ https://www.transparencymarketresearch.com/authorized-generics-market.html
An example of authorized generic drug is Azithromycin Pak which is sold under by the company name Greenstone. Pfizer’s original branded drug Z-pak was approved by the FDA. Before patent expiry of Z-pak, Pfizer allowed Greenstone to sell Z-pak using authorized generic name Azithromycin Pak. Greenstone is a wholly owned subsidiary of Pfizer.
The global authorized generics market is expected to witness strong growth. Authorized generic drugs are priced at significantly discounted rate i.e. 50% to 70% as compared to branded counterparts. Additionally, many of the popular branded drugs of pharma companies are losing patent protection rights, which is also termed as patent cliffs. This would pave the way for entry of new complex generics in the market. These factors are likely to drive the authorized generics market in the near future. Other factors driving the market are health care plans by governments across the world, rapidly increasing cost of branded drugs, and aging populations. On the other hand, possibility of side effects, and lack of regulatory awareness about products and quality management are factors likely to restrain the global authorized generics market.
The global authorized generics market can be classified based on product type, application, end-user, and region.
Obtain the Report Brochure @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=20609
In terms of product type, the global authorized generics market can be segmented into biosimilars, simple generic, super generic, and others. Based on applications the market can be classified into cardiovascular, anti-infective, anti-arthritis, central nervous system, anti-cancer, respiratory, and others.
Geographically, the global authorized generics market can be divided into North America, Asia Pacific, Europe, Latin America, and Middle East & Africa. North America accounted for the largest share of the market, primarily due to technological advancements, rise in demand for generic drugs, increase in overall cost of branded drugs, and presence of key players. Europe held a significant share of the market attributed to advancements in generic drugs, rise in various types of cancer, and blood related disorders. Asia Pacific, however, has been exhibiting high growth rate on account of growing demand for generic drugs, rise in geriatric population, increase in disposable income, and government initiatives to support generic drugs. Rapidly rising population in the region has increased demand for better health care and induced both private and government players to meet the demand. Developing economies such as India and China have also made a significant contribution to the rise of the global authorized generics market by focusing on establishing a better health care infrastructure.
Key players in the market are Teva Pharmaceuticals, Sandoz, Allergan, Mylan, Sun Pharmaceuticals, and STADA Arzneimittel. Other prominent vendors in the market are Abbott, Amgen, Apotex, Aspen, AstraZeneca, Aurobindo Pharma, Baxter, Berlin-Chemie, Biocon, Biogen, Boehringer Ingelheim, Celltrion, Cipla, Coherus Biosciences, Dr. Reddy's Laboratories, Daiichi Sankyo, Eli Lilly and Company, Emcure Pharmaceuticals, Eurofarma Laboratories, Gedeon Richter, Gilead Sciences, and GlaxoSmithKline.
Request for Report TOC @ https://www.transparencymarketresearch.com/sample/sample.php?flag=T&rep_id=20609
About Us
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants, use proprietary data sources and various tools and techniques to gather, and analyze information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact Us
Transparency Market Research,
90 Sate Street, Suite 700,
Albany, NY 12207
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: https://www.transparencymarketresearch.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Authorized Generics Market Plying for Significant Growth During 2024 here
News-ID: 1222448 • Views: …
More Releases from Transparency Market Research

Medium Voltage Fuse Market Outlook 2031: Global Market to Reach US$ 1.8 Billion …
The global medium voltage fuse market is steadily transitioning from a traditional grid-protection niche to a strategic enabler of modern power systems. Rising investments in renewable energy integration, large-scale electrification programs, and infrastructure upgrades are reshaping demand patterns worldwide. Medium voltage fuses-typically rated between 1 kV and 35 kV-are no longer viewed as passive safety components; instead, they are increasingly recognized as critical assets for grid stability, asset protection, and…
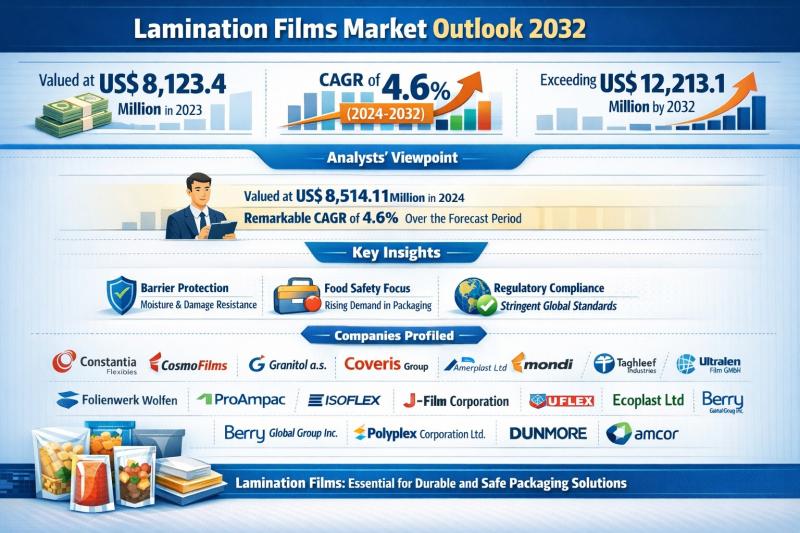
Lamination Films Market Outlook 2032: Global Industry Size to Surpass US$ 12.21 …
The global lamination films market was valued at US$ 8,123.4 million in 2023 and is forecast to reach US$ 8,514.1 million in 2024. Over the forecast period from 2024 to 2032, the market is projected to expand at a compound annual growth rate (CAGR) of 4.6%, ultimately exceeding US$ 12,213.1 million by 2032. This steady growth trajectory reflects the indispensable role of lamination films in modern packaging ecosystems across food,…

Global Curcumin Market Outlook 2031: Natural Antioxidant Demand, Regional Growth …
The global curcumin market is entering a structurally strong growth phase, underpinned by rising consumer preference for natural, plant-based ingredients and increasing clinical validation of curcumin's antioxidant and anti-inflammatory properties. As consumers shift away from synthetic additives and chemical-based therapeutics, curcumin is emerging as a high-value bioactive ingredient across , functional foods, cosmetics, and pharmaceuticals. Premiumization trends in organic products, combined with regulatory validation from food safety authorities, are expected…

Hemoglobin A1c Testing Devices Market to be Worth More Than USD 3.3 Bn by 2034 - …
The global Hemoglobin A1c (HbA1c) Testing Devices Market was valued at US$ 1.4 Bn in 2023 and is projected to expand at a CAGR of 7.7% from 2024 to 2034, reaching more than US$ 3.3 Bn by the end of 2034. The market growth is primarily attributed to the increasing global burden of diabetes, growing awareness about disease management, and technological advancements in diagnostic devices.
Get a concise overview of key…
More Releases for Authorized
Finsmart Accounting Becomes Zoho Authorized Partner
Leading accounting outsourcing company of India becomes Zoho partner to help businesses make the most of its comprehensive finance and accounting solutions.
Pune, India May 05, 2023 - Finsmart Accounting is known globally for offering offshore bookkeeping services in India. The company recently announced its partnership with Zoho to help organizations make the most of Zoho accounting and finance solutions.
Zoho's software and web tools are used by millions of…
CRM Masters Authorized Zoho Consulting Partner
Founded in 2016, CRM Masters Infotech is a Zoho Premium Partner and a Salesforce Certified Partner.CRM Masters is a trusted source for businesses to employ digital solutions with ultimate security. As Premium Zoho Consulting Partners, we offer exceptional and comprehensive implementation of the entire Zoho One suite - from CRM Plus and Books to People Plus & Creator- guaranteeing full GDPR & HIPPA compliance at all times! Unlock your business's…
Matrox adds ASI Canada as an Authorized Distributor
Partnership allows ASI to offer a wide range of graphics solutions to the Canadian market
MONTREAL — Nov. 7, 2013 — Fremont, California-based ASI Computer Technologies, a leading distributor of computer components and peripheral products to over 20,000 VARs throughout North America with branch locations in Toronto, Montreal, and Vancouver, has recently been added as a Matrox Graphics Authorized Distributor in Canada.
ASI has been an authorized distributor in the United States…
Palmsol Joins Google Apps Authorized Reseller Program
November 11, 2011-- Palmsol today announced it has become an authorized reseller of the Google Apps™ suite of communication and collaboration tools.
“The Google Apps Reseller program will help us enhance the value of Google Apps for businesses in South Florida," said Michael Zuloaga, managing partner of Palmsol – IT Solutions for Business. "Use of cloud computing, through solutions like Google Apps, is one way to innovate traditional…
Crowne Ventures To Reduce Number Of Authorized Shares
Gold mining and exploration growth stock company, Crowne Ventures, Inc. (Stock Symbol: CRWV), has announced that its Board of Directors and Stockholders have approved an Amendment to the Articles of Incorporation to reduce the number of authorized shares in the Company from 1,000,000,000 to 550,000,000, with 500,000,000 common shares authorized, and 50,000,000 preferred shares authorized.
In addition, the Company has announced the launch of its new website: http://www.CrowneMining.com.…
ASI Creates Authorized iMIS Fundraising Partner Program
ALEXANDRIA, Va., (June 8, 2011)—Advanced Solutions International (ASI), a leading global provider of web-based software for donor-based non-profits, today announced the company has accredited several solution providers and consultants for its global Authorized iMIS Fundraising Partner program based on the quality of their performance serving donor-based organizations. The organizations that have been accredited are existing authorized partners and were selected for this program as a result of their success and…
