Press release
Ease in FDA clearance is the factor driving growth of the global arthroscopy procedures and products market currently
The global arthroscopy procedures and products market is set to expand at 5.7% CAGR through 2023, according to “Arthroscopy Procedures and Products Market: Global Industry Analysis and Opportunity Assessment, 2015 - 2023”, a new report by Future Market Insights.The global minimally invasive surgery devices market is forecast to surpass US$ 30 Bn by 2018, and the growth is expected to rub off on the arthroscopy market. Increasing application in sports medicine, demographic trends, rising prevalence of osteoarthritis and ease in FDA clearance are key factors driving growth of the global arthroscopy procedures and products market. On the other hand, risk of edema due to irrigation fluids and increasing pricing pressures are anticipated to hamper market growth during the forecast period.
A sample of this report is available upon request @ https://www.futuremarketinsights.com/reports/sample/rep-gb-1386
Arthroscopy Procedures and Products Market Segmentation
By product type, the global arthroscopy procedures and products market is segmented into arthroscopes and visualisation systems, arthroscopic resection systems, arthroscopic fluid management systems, arthroscopic implants, arthroscopy radiofrequency (RF) systems, arthroscopic drills and fixation systems and other arthroscopy instruments and accessories.
The arthroscopic implants segment is further sub-segmented into knee implants, shoulder implants, hip implants and other implants. The arthroscopic implants product type segment accounts for highest share of the arthroscopy procedures and products market currently, and is expected to remain the leading segment in terms of value during the forecast period. Increase in clinical evidences related to the safety, efficacy and economic benefits of arthroscopic implants in younger patients is expected to boost growth of arthroscopic implants segment during the forecast period.
By procedure type, the knee arthroscopy segment is expected to hold more than half the global market share, and the revenue from the segment is projected to expand at the highest CAGR over the forecast period as compared to other segments.
Browse the full "Arthroscopy Procedures and Products Market: Global Industry Analysis and Opportunity Assessment 2015 - 2023" market research report at https://www.futuremarketinsights.com/reports/arthroscopy-procedures-products-market
By region, North America is the largest market in terms of value currently and is expected to retain its position through the forecast period. Increasing number of ambulatory surgical centres, developing techniques and equipment, and increasing usages of arthroscopic instruments used in hips, shoulders, ankles and wrists procedures are factors expected to boost growth of the North America market over the forecast period.
Markets in the Americas and Europe collectively accounted for over 75% revenue share of the global arthroscopy procedures and products market in 2015. The market in APAC region is projected to exhibit an above average CAGR in terms of value during the forecast period from 2015 to 2023. Increase in preference of arthroscopic surgeries and technological advancement in arthroscopic instruments are factors expected to fuel demand for arthroscopy products over the forecast period.
To view TOC of this report is available upon request @ https://www.futuremarketinsights.com/askus/rep-gb-1386
Key market players covered in this report are Arthrex, Inc., CONMED Corporation, Johnson & Johnson, KARL STORZ GmbH & Co. KG, Olympus Corporation, Richard Wolf GmbH and Stryker Corporation. Collaboration with partners to maintain market leadership in sports medicine in different regions, engaging in R&D activities that focus on orthopaedic surgical treatments, leveraging core expertise to strengthen business plans and acquisitions to strengthen the arthroscopy manufacturing business units in order to enhance market foothold over the next four to five years are the key strategies adopted by the arthroscopy products manufacturers.
About Us
Future Market Insights is the premier provider of market intelligence and consulting services, serving clients in over 150 countries. FMI is headquartered in London, the global financial capital, and has delivery centres in the U.S. and India.
FMI’s research and consulting services help businesses around the globe navigate the challenges in a rapidly evolving marketplace with confidence and clarity. Our customised and syndicated market research reports deliver actionable insights that drive sustainable growth. We continuously track emerging trends and events in a broad range of end industries to ensure our clients prepare for the evolving needs of their consumers.
Contact Us
U.S. Office
616 Corporate Way, Suite 2-9018,
Valley Cottage, NY 10989,
United States
T: +1-347-918-3531
F: +1-845-579-5705
Email: sales@futuremarketinsights.com
Web:https://www.futuremarketinsights.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Ease in FDA clearance is the factor driving growth of the global arthroscopy procedures and products market currently here
News-ID: 1146810 • Views: …
More Releases from Future Market Insights
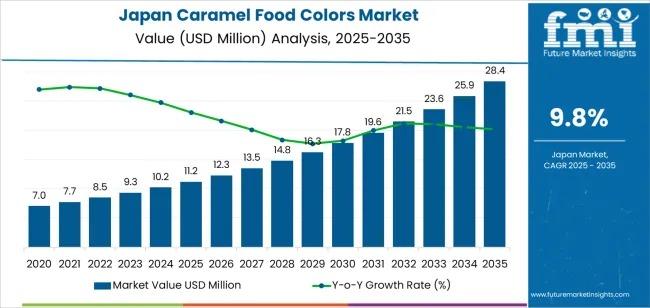
Japan Caramel Food Colors Industry Outlook to 2036: Strategic Insights for R&D, …
The Japanese caramel food colors market is on a steady growth trajectory, with demand projected to rise from USD 11.2 million in 2025 to USD 28.4 million by 2035, registering a CAGR of 9.8%. The initial phase of the forecast period (2025-2030) anticipates a steady increase in demand, reaching approximately USD 17.8 million by 2030, driven by the expanding use of caramel colors across confectionery, dairy, and baked goods.
The market's…
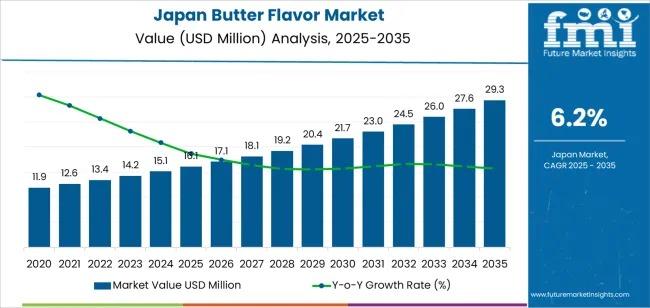
Comprehensive Analysis of the Japan Butter Flavor Market: Technology Evolution, …
The demand for butter flavor in Japan is projected to rise from USD 16.1 million in 2025 to USD 29.4 million by 2035, reflecting a steady compound annual growth rate (CAGR) of 6.2%. This growth is underpinned by increasing adoption across bakery products, confectionery items, and dairy-based preparations, as manufacturers seek to enhance taste experiences and deliver authentic dairy character in a wide range of food offerings.
The Japanese bakery and…
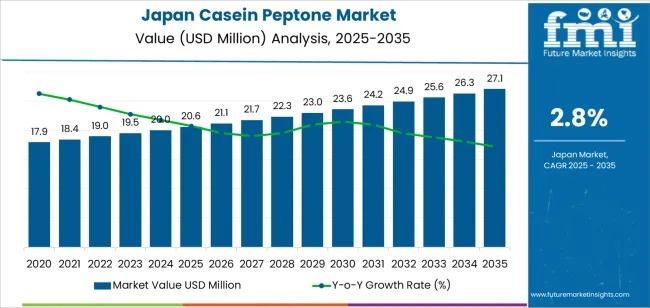
Japan Casein Peptone Market Deep-Dive 2026-2036: Strategic Forecasts, Market Ent …
The demand for casein peptone in Japan is projected to grow steadily, reaching USD 27.1 million by 2035, up from USD 20.6 million in 2025, reflecting a compound annual growth rate (CAGR) of 2.8%. During the early forecast period (2025-2030), demand is expected to rise from USD 20.6 million to approximately USD 23.6 million, supported by its widespread applications in biotechnology, pharmaceuticals, and food industries. Casein peptone continues to play…

Global Boride Powder Market Size, Share & Forecast: High-Growth Segments, Value …
The global boride powder market is valued at USD 19.7 billion in 2025 and is projected to reach USD 32.2 billion by 2035, advancing at a steady 5.0% CAGR over the forecast period. This upward trajectory reflects increasing adoption of boride-based compounds in aerospace technology, high-temperature processing environments, and advanced coating applications, where exceptional thermal stability, corrosion resistance, and mechanical strength are essential for operational performance and product reliability.
Key Market…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…
