Press release
Biosimilar Monoclonal Antibodies Market Key Manufacturers, Development Trends And Competitive Analysis 2025
Biosimilar monoclonal antibodies can be defined as biological molecules derived from living cells or organisms. These are large complex molecules. Biosimilars are developed after the expiry of patent of approved recombinant drugs, and are also known as biologics. These biologics display a certain level of variability which can be attributed to the variations in the biological expression system of the recombinant and the manufacturing process. Biosimilars are regulated by the various governing bodies in different countries. In the U.S., the FDA regulates the release of these biosimilars, which are required to undergo pharmacovigilance regulations as its reference approved monoclonal antibody. In Europe, biosimilars are approved by the European Medicines Agency (EMEA). Manufacturers of biosimilars are required to submit risk management plan in addition to the marketing application.Browse Premium Industry Research Report with Analysis: https://www.transparencymarketresearch.com/biosimilar-monoclonal-antibodies-market.html
Increase in patent expiries of blockbuster monoclonal antibodies has led to a rise in interest among pharmaceutical companies to develop biosimilars. This is projected to drive the market during the forecast period. Focus on research and development contributing to innovative product pipeline and expanding health care infrastructure fuel the growth of the biosimilar monoclonal antibodies market. Additionally, increasing demand for cost effective treatment among patients boosts the growth of the market. Furthermore, launch of biosimilars owing to the patent expiries of Johnson & Johnson’s Remicade, Roche’s Herceptin, and Abbott’s Humira is one of the major factors propelling the market. However, stringent regulations related to product approvals and cost structure associated with the manufacturing process are likely to hamper market growth during the forecast period. Furthermore, lack of clarity related to the regulations concerning the development of biosimilar monoclonal antibodies in various emerging markets is expected to act as a restraint of the market.
The global biosimilar monoclonal antibodies market can be segmented based on indication, molecule, distribution channel, and region. In terms of indication, the market can be categorized into autoimmune diseases, oncology, hematology, gastrointestinal diseases, and others. Based on molecule, the biosimilar monoclonal antibodies market can be classified into bevacizumab, trastuzumab, infliximab, rituximab, abciximab, and adalimumab. The rituximab and infliximab segments are anticipated to dominate the market during the forecast period. Oncology was the dominant segment in 2016 and the trend is anticipated to continue in the next few years. In terms of distribution channel, the market can be divided into hospital pharmacies, retail pharmacies, specialty pharmacies, and others. The retail pharmacies and others segments, which includes online pharmacies, are anticipated to record substantial growth during the forecast period.
Request for Sample Copy of Report: https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=32336
Geographically, the global biosimilar monoclonal antibodies market can be segmented into five regions: North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America accounted for the highest share of the global market in terms of revenue in 2016 and the trend is anticipated to continue during the forecast period. Strong growth in the region can be attributed to increase in product approvals, well-established health care infrastructure, and favorable reimbursement scenario. Europe is expected to be the second leading market for biosimilar monoclonal antibodies. However, the sluggish economy in the region is likely to negatively impact the growth of the market. The market in Asia Pacific is anticipated to record a significantly high CAGR owing to factors such as increasing patient population, growing government focus on enhancing health care facilities, and increase in product approvals in Japan.
Leading players operating in the global biosimilar monoclonal antibodies market are Biocon Limited, Allergan plc, Accord Healthcare Ltd., Boehringer Ingelheim GmbH, 3SBio, Inc., Novartis AG, Amgen, Inc., Alvartis Pharma, Pfizer, Inc., Celltrion, Alfred E. Tiefenbacher (GmbH & Co. KG), Hospira, and Dr. Reddy's Laboratories, among others.
Request Table of Content of Report: https://www.transparencymarketresearch.com/sample/sample.php?flag=T&rep_id=32336
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector – such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports strive to provide clients to serve their overall research requirement.
Contact US:
Transparency Market Research,
90 State Street,
Suite 700,
Albany NY – 12207
United States
Tel: +1-518-618-1030
Email: sales@transparencymarketresearch.com
Website: https://www.transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilar Monoclonal Antibodies Market Key Manufacturers, Development Trends And Competitive Analysis 2025 here
News-ID: 972058 • Views: …
More Releases from Transparency Market Research
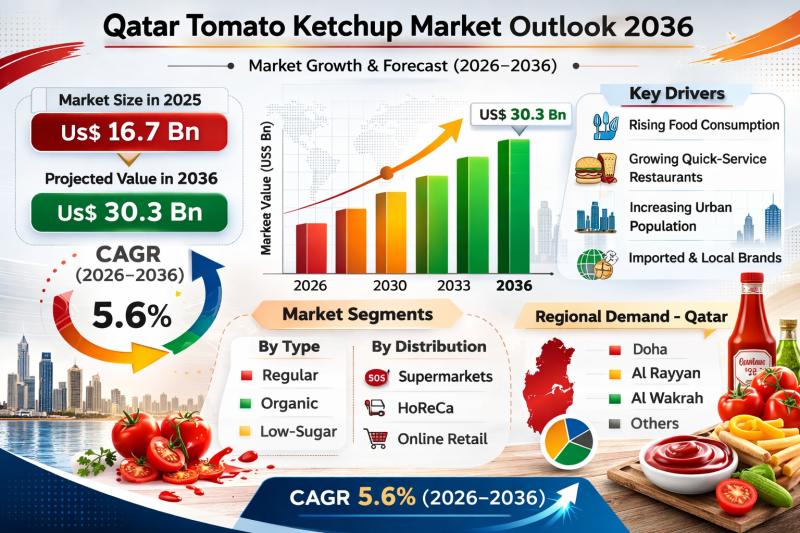
Qatar Tomato Ketchup Market Expanding at 5.6% CAGR Through 2036 - By Type / By P …
The Qatar Tomato Ketchup Market was valued at US$ 16.7 Bn in 2025 and is projected to reach US$ 30.3 Bn by 2036, expanding at a CAGR of 5.6% from 2026 to 2036. The steady growth trajectory reflects increasing consumption of convenience foods, rising demand from quick-service restaurants (QSRs), and continued innovation in product variants and packaging formats.
Get a concise overview of key insights from our Report in this sample…
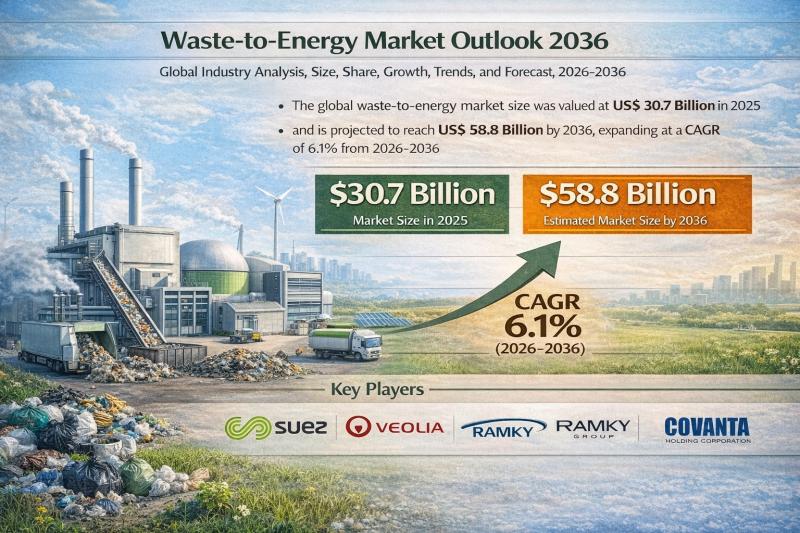
Waste-to-Energy Market to Reach US$ 58.8 Billion by 2036 at 6.1% CAGR | Transpar …
The global waste-to-energy (WtE) market is entering a dynamic growth phase as governments and industries intensify efforts to transition toward sustainable waste management and renewable power generation. Waste-to-energy refers to the process of converting municipal solid waste (MSW), agricultural waste, and other refuse into usable forms of energy such as electricity, heat, and fuel through technologies including incineration, gasification, pyrolysis, and anaerobic digestion.
As environmental pressures mount and landfill capacity shrinks,…
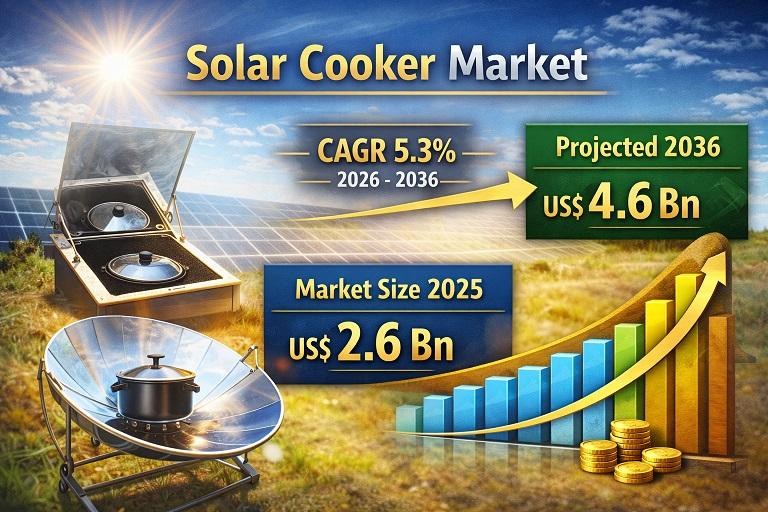
Solar Cooker Market to Reach USD 4.6 Billion by 2036, Driven by Rising Demand fo …
The Solar Cooker Market is witnessing steady expansion as global demand for sustainable and clean cooking solutions accelerates. Solar cookers, which utilize sunlight as a renewable heat source for food preparation, are gaining recognition as eco-friendly alternatives to conventional cooking methods that rely on fossil fuels, firewood, and electricity. Rising environmental awareness, increasing fuel costs, and government initiatives promoting renewable energy adoption are key factors supporting market growth worldwide.
The Solar…
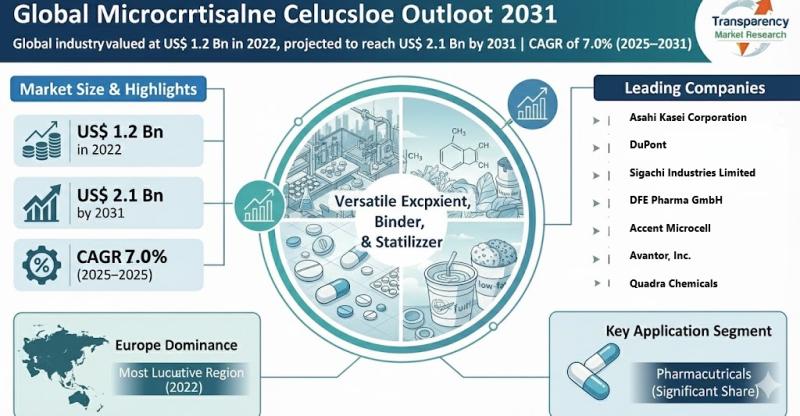
Microcrystalline Cellulose Market to Reach US$ 2.1 Bn by 2031, Driven by Pharmac …
The global microcrystalline cellulose (MCC) market was valued at US$ 1.2 Bn in 2022 and is projected to reach US$ 2.1 Bn by the end of 2031, expanding at a CAGR of 7.0% from 2023 to 2031. The market is expected to witness robust growth driven by increasing demand from the pharmaceutical and food & beverage industries, expanding applications in personal care products, and rising consumer preference for clean-label and…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
