Press release
Remicade Biosimilar Market Research Study for the Period 2016-2026
Biosimilar can be defined as a type of biological product that is highly similar to an already FDA approved drug, known as reference biological product. Biosimilars are drugs licensed by U.S. FDA and European Medicine Agency (EMA) and reflect no clinical and meaningful differences from the reference products in terms of safety, purity, efficacy and effectiveness. These biosimilar drugs can only be approved for the indications and conditions that have been previously approved for the reference product by big regulatory agencies.Remicade (infliximab) is a monoclonal antibody originally produced by Janssen Biotech Inc., and Merck & Co. (MSD) in partnership and licensed by the U.S. FDA in 1998. It is used in the treatment of Crohn’s disease in both adult and pediatric patients. Additionally it is also used for treatment of active ulcerative colitis, moderate to severe rheumatoid arthritis in combination with methroxate, spinal and active psoriatic arthritis, and plaque psoriasis. The U.S. FDA has approved a biosimilar drug similar to Remicade, named Inflectra, on 05 April, 2016, which is expected to erode the market share of Remicade due to lower competitive pricing. Inflectra (infliximab dyyb) is sold in the European market, after receiving an approval from the EMA’s CHMP in June, 2013 under the brand name Remsima (developed by South Korea’s Celltrion Healthcare and marketed by Pfizer’s Hospira). The drug Remsima is sold at a discount of 30% than that of original Remicade in 11 European markets including UK, France, Germany and Italy. Another Japanese company, Nippon Kayaku launched Infliximab BS in Japan on 28 November 2014 – however, the Japanese license only covers Crohn’s disease, rheumatoid arthritis and ulcerative colitis indications. The U.S. FDA has now launched Remicade’s biosimilar Inflectra, which is only the second biosimilar drug to be approved by the agency.
Request Report Sample@ https://www.futuremarketinsights.com/reports/sample/rep-gb-1413
Drivers and Restraints:
Drivers for the Remicade biosimilar include rising incidence of autoimmune diseases particularly rheumatoid arthritis and plaque psoriasis, early patent expiry of the branded version, discounted pricing across the European market and faster reaction times due to intravenous mode of administration. Further, entry of biosimilar version could provide financial relief on healthcare systems and improve patient’s accessibility to essential medication.
Barriers of the Remicade biosimilar include serious side effects associated with the use of drug that could lead to hospitalization or even be fatal. These include tuberculosis, bacterial sepsis, invasive fungal infections (such as histoplasmosis) and others. As such, manufacturer of the drug has been mandated to include a “Boxed Warning” to alert both healthcare professionals and patients. Further, complex nature of the molecule and lack of FDA approved facilities for manufacturing the drug are factors that could restrain the growth of the drug’s market in developing regions.
Market Segmentation:
The Remicade (infliximab) biosimilar market is segmented based on approved disease indications and regions.
By Disease Indication
Crohn’s disease
Rheumatoid arthritis
Ankylosing spondylitis
Psoriatic arthritis
Ulcerative Colitis
Plaque psoriasis
By Regions
North America
Western Europe
Eastern Europe
Asia Pacific excluding Japan
Japan
Latin America
MEA
Visit For TOC@ https://www.futuremarketinsights.com/toc/rep-gb-1413
Market Overview:
Early loss of patent exclusivity is one the major factors that could fuel attractive market growth of the Remicade biosimilar over the forthcoming years. Rising prevalence of relevant autoimmune disorders coupled with discounted pricing of the Remicade biosimilar in European markets are factors expected to contribute to increased referral and consumption of the drug. Development of faster approval procedures and proper U.S. FDA approved manufacturing facilities in the regional nodal countries are factors that contribute to increased drug uptake. Further, distinct naming and transparent labeling to ensure correct prescribing and dispensing and enhanced post-marketing surveillance are factors that could contribute largely towards prescriber confidence, and enhanced market uptake of the drug over the coming years. However, safety issues concerning manufacturing facilities along with potential side effects of drug consumption could hamper for acceptance of infliximab biosimilar over the long run.
Remicade Biosimilar Market: Region- wise Outlook:
Depending on geographic regions, global Remicade biosimilar market is segmented into seven key regions: North America, South America, Eastern Europe, Western Europe, Asia Pacific excluding Japan, Japan and Middle East & Africa.
In terms of geography, Europe dominates the Remicade biosimilar market, followed by Japan and Latin America. The prime reason for the same is the launch of the biosimilar version soon after the patent expiry of the branded version. However, systematic and faster drug review process is expected to create revenue traction in markets over North America and other regions. Stringent regulatory approval procedures and streamlined manufacturing guidelines, particularly in the Central and South American nations, could lead to development of effective regional manufacturing and distribution strategies for Remicade biosimilars. Finally, rising government support for development of biosimilar drugs and low switching tendency from physicians secure the future market growth of the biosimilar in the near term.
Remicade Biosimilar Market: Key Players:
Some of the key market players in Remicade (infliximab – mAb) market are Janssen Biotech Inc., Merck &Co., Pfizer Inc. (AC. Hospira), Celltrion Inc., Alvogen, Napp Pharmaceuticals, and Nippon Kayaku.
About Us –
Future Market Insights (FMI) is a leading market intelligence and consulting firm. We deliver syndicated research reports, custom research reports and consulting services, which are personalized in nature. FMI delivers a complete packaged solution, which combines current market intelligence, statistical anecdotes, technology inputs, valuable growth insights, an aerial view of the competitive framework, and future market trends.
CONTACT:
616 Corporate Way, Suite 2-9018,
Valley Cottage, NY 10989,
United States
T: +1-347-918-3531
F: +1-845-579-5705
Email: sales@futuremarketinsights.com
Website: www.futuremarketinsights.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Remicade Biosimilar Market Research Study for the Period 2016-2026 here
News-ID: 945874 • Views: …
More Releases from Future Market Insights
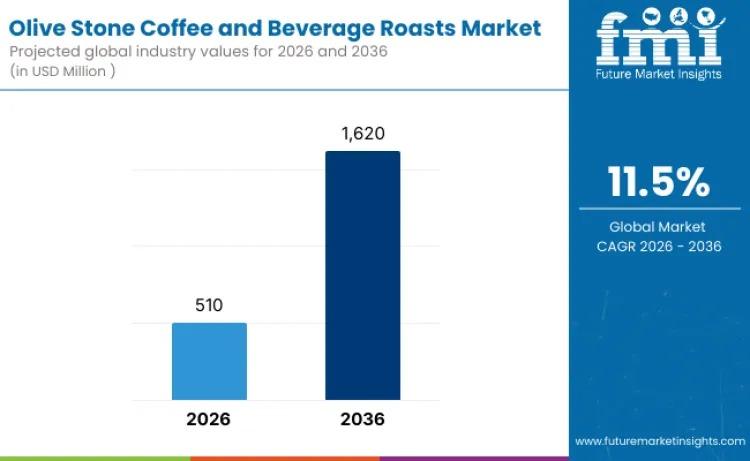
Global Olive Stone Coffee and Beverage Roasts Market to Reach USD 1,620 Million …
The global olive stone coffee and beverage roasts market is entering a high-growth decade, fueled by sustainability innovation and evolving specialty coffee culture. Valued at USD 510 million in 2026, the market is projected to reach USD 1,620 million by 2036, expanding at a compelling CAGR of 11.5%.
As consumers increasingly seek beverages that combine sustainability, functionality, and distinctive taste, olive stone-based roasting solutions are transitioning from niche experimentation to structured…
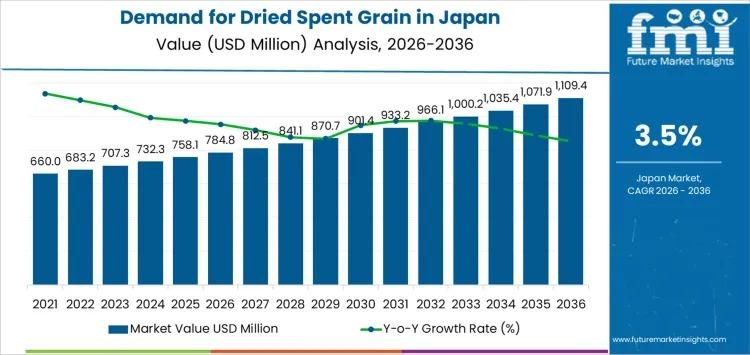
Japan Dried Spent Grain Market to Surpass USD 1.1 Billion by 2036 as Feed Optimi …
Japan's dried spent grain market is entering a decade of steady, value-driven expansion, supported by structured feed demand, brewery byproduct utilization, and rising integration of fiber-rich ingredients into food manufacturing. Industry estimates place the market at USD 784.8 million in 2026, with projections indicating growth to USD 1,109.4 million by 2036, reflecting a CAGR of 3.5%.
Between 2020 and 2026, demand increased from USD 637.5 million to USD 784.8 million, shaped…
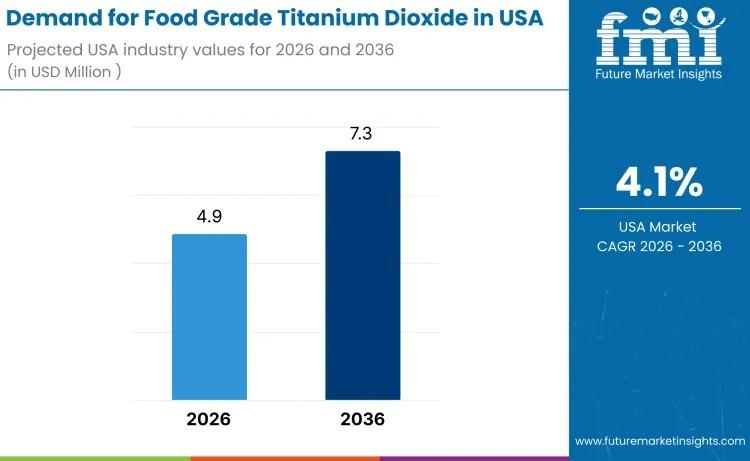
USA Food Grade Titanium Dioxide Market to Reach USD 7.3 Million by 2036 Amid Ste …
The demand for food grade titanium dioxide in the USA is valued at USD 4.9 million in 2026 and is projected to reach USD 7.3 million by 2036, expanding at a CAGR of 4.1%. Growth remains moderate yet stable, supported by continued use of titanium dioxide as a whitening and opacifying agent across confectionery coatings, bakery decorations, sauces, dairy analogues, and processed food matrices.
Despite heightened regulatory scrutiny and evolving clean-label…
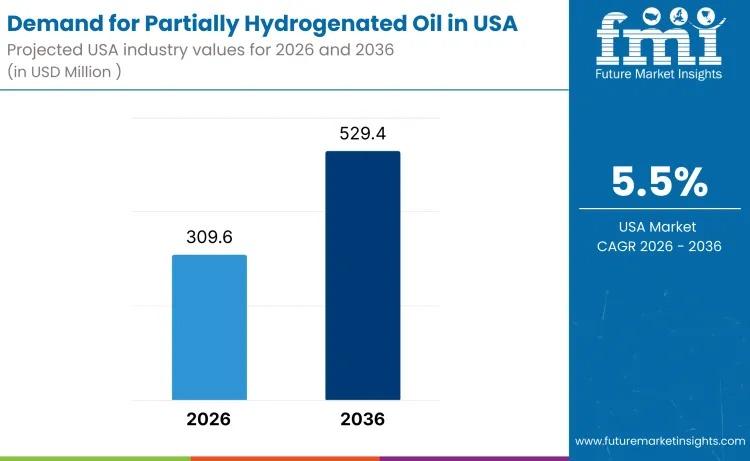
USA Partially Hydrogenated Oil Market to Reach USD 529.4 Million by 2036 Amid Me …
The demand for partially hydrogenated oil in the USA is projected to rise from USD 309.6 million in 2026 to USD 529.4 million by 2036, expanding at a steady CAGR of 5.5%. While edible applications remain tightly regulated, demand persists across specialty industrial and permitted food-related segments where oxidative stability, viscosity control, and texture performance remain critical.
Despite regulatory constraints on trans fats in conventional food manufacturing, PHOs continue to serve…
More Releases for Remicade
Surge In Autoimmune Diseases Fuels Remicade Biosimilar Market Growth Emerges as …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
What Is the Expected CAGR for the Remicade Biosimilar Market Through 2025?
In recent times, we've seen significant expansion in the remicade biosimilar market. Projected growth from 2024's $3.95 billion to $4.89 billion in 2025 indicates a compound annual growth rate (CAGR) of 23.8%. This rise during the historic…
Remicade Biosimilar Market Size, Trends, Analysis And Forecast 2024-2033
"The Business Research Company recently released a comprehensive report on the Global Remicade Biosimilar Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive Sample…
Affordable Innovation: 2024 Global Market Report on Remicade Biosimilars
The Business Research Company has recently revised its global market reports, now incorporating the most current data for 2024 along with projections extending up to 2033.
Remicade Biosimilar Global Market Report 2024 by The Business Research Company offers comprehensive market insights, empowering businesses with a competitive edge. It includes detailed estimates for numerous segments and sub-segments, providing valuable strategic guidance.
The Market Size Is Expected To Reach $9.1 billion In 2028 At…
Remicade Biosimilar Market Size & Growth Analysis Report, 2021-2027
Biosimilar can be defined as a type of biological product that is highly similar to an already FDA approved drug, known as reference biological product.
(Get 15% Discount on Buying this Report)
Get Sample Copy of Remicade Biosimilar Market at: https://www.orionmarketreports.com/remicade-biosimilar-2-market/48410/#ert_pane1-1
Segment by Type
• 100mg/10ml
• 500mg/50ml
Segment by Application
• Blood Disorders
• Oncology Diseases
By Company
• Synthon Pharmaceuticals
• LG Life Sciences
• Novartis (Sandoz)
• Celltrion
• Biocon
• Hospira
• Merck Serono (Merck Group)
• Biogen idec Inc
A full report of Global Remicade Biosimilar Market is available at: https://www.orionmarketreports.com/remicade-biosimilar-2-market/48410/
Scope of the Report
The research…
Biosimilar of Remicade Market Is Thriving Worldwide
In a recent S&R Research publish Global Biosimilar of Remicade Market Insights, Forecast to 2025, analysts provide an in-depth analysis of the global market for Biosimilar of Remicade. By analyzing its historical and forecast data, the analysis analyzes the different aspects of the market. Some are part of the coverage and are the core and emerging players being profiled Synthon Pharmaceuticals, LG Life Sciences, Novartis (Sandoz), Celltrion, Biocon, Hospira, Merck…
Remicade (Infliximab) Biosimilar Clinical Trial Insight
“Remicade (Infliximab) Biosimilar Clinical Trial Insight” report by PNS Pharma gives comprehensive clinical insight on 17 biosimilar version of Remicade drug in clinical pipeline. Currently there are 2 biosimilars in Phase-III trials and are expected to be commercially available in next 5-8 years. Currently 3 biosimilar version of Remicade are commercially available in India, Brazil and European countries for the treatment of Ankylosing spondylitis, Crohn's disease and Rheumatoid Arthritis. The…
