Press release
Biosimilars Insulin Market: Comprehensive Evaluation of the Market Via In-Depth Qualitative Insights
Biosimilars is a biological product designed to have similar active properties to the one that has been previously licensed and has no clinically significant differences in terms of safety and effectiveness. Biosimilars insulin is a type of biosimilars where it is indistinguishable to the reference insulin product and is already been approved by FDA or licensed. The producers of biosimilars use the similar manufacturing techniques as of the patented product but not likely identical to that used by the patent holder. Due to increasing insulin manufacturers from the developed and developing countries, the patents for insulin formulations had neared expiry or were ended. This created a necessity to seek approvals on biosimilar insulin for the not yet established companies in the highly regulated markets such as Europe and United States. In 2014, The European Commission (EC) first granted insulin treatment through the biosimilars pathway to Eli Lilly and Company and Boehringer Ingelheim GmbH. Lilly/Boehringer Ingelheim developed a biosimilar insulin called “Insulin glargine” and was the fourth diabetes product which was approved from Lilly-Boehringer Ingelheim Alliance in Europe. Basaglar is the first “biosimilar” insulin product to be approved and launched in U.S. which was developed by the alliance of Lilly/Boehringer Ingelheim.Biosimilar insulin proved to have an effective treatment for patients suffering from diabetes mellitus by reducing the cost for the treatment. There are some companies who have developed devices which allow the safe and less painful delivery of biosimilar insulin thus making the device a market differentiator even though it’s chemically equivalent to the branded product. Many companies in the emerging economies have started developing biosimilar insulin due to the favorable regulatory requirements as compared to the companies in developed countries where their stringent regulatory requirements. As the regulatory requirements for the approval process of biosimilars are considerably higher than those required for the generic drugs and entail the comprehensive analysis for each of its characteristics.
Request Report For TOC @ https://www.persistencemarketresearch.com/toc/11674
Biosimilars have increased the opportunities in healthcare services and have decreased the expenditures creating a significant competition between the companies manufacturing branded products and biosimilars. Globally, the major factors responsible for surging the demand for biosimilars insulin in the market are increase in diagnosed and undiagnosed diabetes, patent expiry for insulin, growing population. Diabetes is considered to be one of the fastest growing disease and attribute to 3 million deaths annually, and has become a pandemic. Increasing poverty level in developing countries has created a pressure on the healthcare industry to develop cost effective products and treatment options especially for diabetes patients. The only aspects which can affect the biosimilars market are safety and efficacy of new biosimilars also the stringent regulatory bodies leading to delayed approvals.
According to the Biosimilars Pipeline Database, there are total 1,357 product entries for biobetters and biosimilars constitute 737 of them (March, 2016). Among the top targeted reference products, insulin and analogs constitute 51 and the companies producing it include Biocon, Mylan N.V., Sanofi, Novo Nordisk and others. Sanofi, a multinational pharmaceutical company is also developing LixiLan, a mix of basal insulin with GLP-1 which is directed to treat for type 2 diabetes. Eli Lilly and Company, a U.S. based company is currently running Phase III clinical trials for a non-insulin drug Empagliflozin (BI10773) targeting the treatment for type 1 diabetes.
Request to Sample of Report @ https://www.persistencemarketresearch.com/samples/11674
The new trend in the market is developing ultra-rapid acting insulins to replace Humalog and Novolog (patents expired) which is important for pump users and disorder related to real-time artificial pancreas. There are new attempts made by companies to create oral insulin such as ingestible capsules for better drug delivery. Most of the biosimilars have reached Phase III and are soon to get approved by the respective regulatory bodies and will reach the market with cheaper prices as compared to the branded products. Various multinationals are forming alliances and partnerships to develop biosimilar insulin such as Eli Lilly and Company and Boehringer Ingelheim Gmb, Biocon Limited and Pfizer Inc., Mylan N.V. and Momenta Pharmaceuticals.
Pre Book Full Report @ https://www.persistencemarketresearch.com/checkout/11674
About Us
Persistence Market Research (PMR) is a U.S.-based full-service market intelligence firm specializing in syndicated research, custom research, and consulting services. PMR boasts market research expertise across the Healthcare, Chemicals and Materials, Technology and Media, Energy and Mining, Food and Beverages, Semiconductor and Electronics, Consumer Goods, and Shipping and Transportation industries. The company draws from its multi-disciplinary capabilities and high-pedigree team of analysts to share data that precisely corresponds to clients’ business needs.
PMR stands committed to bringing more accuracy and speed to clients’ business decisions. From ready-to-purchase market research reports to customized research solutions, PMR’s engagement models are highly flexible without compromising on its deep-seated research values.
Contact
Persistence Market Research Pvt. Ltd
305 Broadway
7th Floor, New York City,
NY 10007, United States,
USA – Canada Toll Free: 800-961-0353
Email: sales@persistencemarketresearch.com
media@persistencemarketresearch.com
Web:www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilars Insulin Market: Comprehensive Evaluation of the Market Via In-Depth Qualitative Insights here
News-ID: 908154 • Views: …
More Releases from Persistence Market Research

Off-Highway Radiators Market to Reach US$ 7.2 Bn by 2033 as Leading Players Like …
The off-highway radiators market plays a vital role in ensuring efficient thermal management in heavy-duty equipment used across construction, agriculture, mining, and forestry sectors. These radiators regulate engine temperatures, prevent overheating, and support consistent equipment performance under extreme operating conditions. Growing mechanization and the expansion of infrastructure projects worldwide are increasing reliance on durable cooling systems. Equipment manufacturers are prioritizing high-performance radiators that offer reliability, longer service life, and resistance…
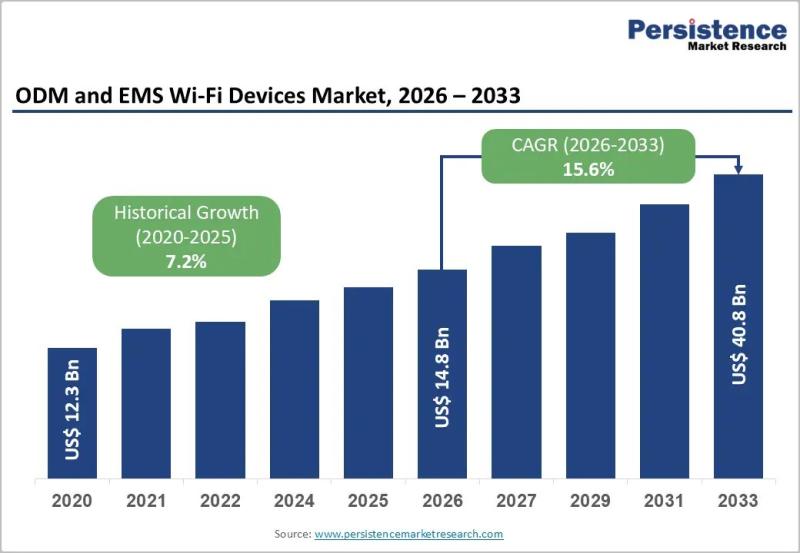
ODM and EMS Wi-Fi Devices Market to Reach US$ 40.8 Billion by 2033 at 15.6% CAGR
The global ODM and EMS Wi-Fi devices market is projected to be valued at US$ 14.8 billion in 2026 and is forecast to surge to US$ 40.8 billion by 2033, registering a robust CAGR of 15.6% between 2026 and 2033. This rapid growth reflects the accelerating demand for advanced wireless connectivity solutions across residential, enterprise, and industrial environments. The expansion of 5G infrastructure, enterprise digital transformation strategies, and large-scale IoT…
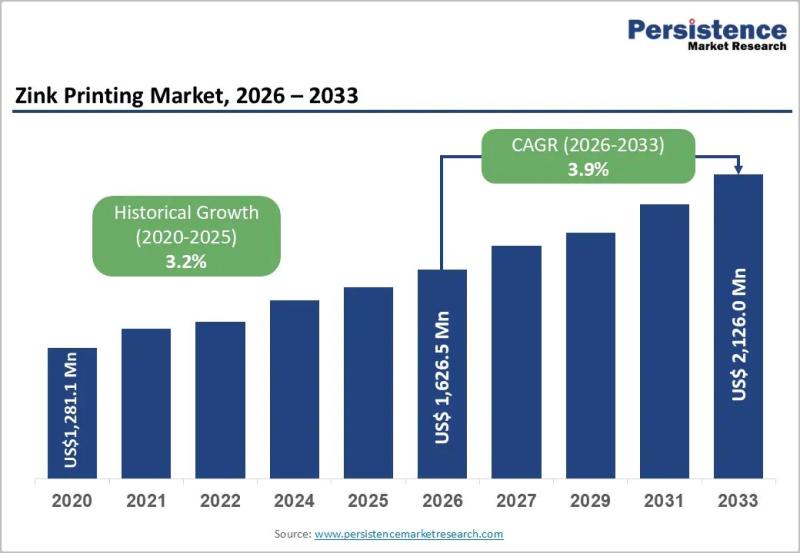
Zink Printing Market to Reach US$ 2,126.0 Million by 2033 at 3.9% CAGR
Zink Printing Market Size and Trends Analysis
The global Zink printing market is projected to be valued at US$ 1,626.5 million in 2026 and is expected to reach US$ 2,126.0 million by 2033, expanding at a CAGR of 3.9% between 2026 and 2033. Zink (Zero Ink) printing technology eliminates the need for ink cartridges by using heat-activated color crystals embedded within specialized paper. This innovation has positioned Zink printers as a…
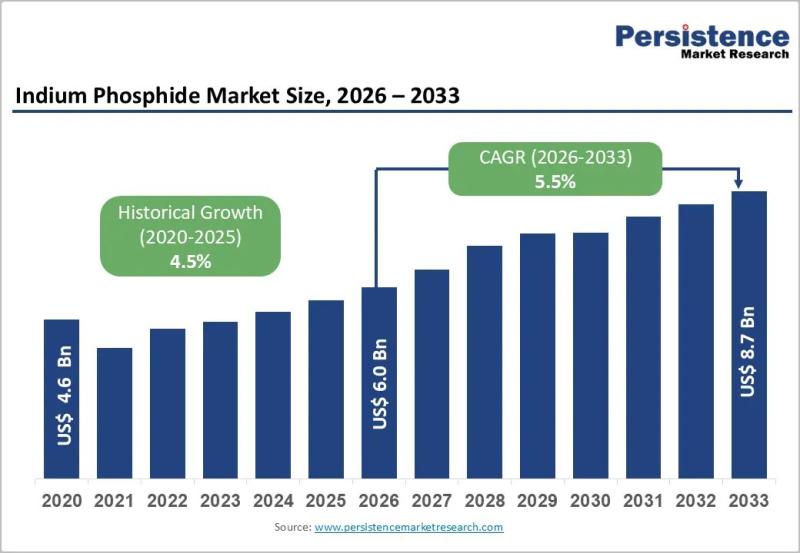
Indium Phosphide Market to Reach US$ 8.7 Billion by 2033 at 5.5% CAGR
The global Indium Phosphide (InP) market is poised for steady expansion, with its valuation expected to reach US$ 6.0 billion by 2026 and further grow to US$ 8.7 billion by 2033, registering a CAGR of 5.5% between 2026 and 2033. Indium phosphide, a high-performance compound semiconductor material, is widely used in optoelectronics, high-frequency electronics, and photonic integrated circuits (PICs). The market growth is largely fueled by the accelerating deployment of…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
