Press release
Advanced Therapy Medicinal Products Market Key Growth Factors and Forecast Upto 2025
Advanced therapy medicinal products (ATMPs) are innovative therapies that are based on gene therapy, somatic cell therapy, and tissue-built products. ATMPs are drug products which leverage living cellular or active genetic materials to offer novel treatment modalities in a range of both acute and chronic diseases. These therapies are relied upon for vital medical advantages. The advanced therapies are revolutionary treatments for a number of diseases or injuries, such as skin in burns victims, Alzheimer's, cancer, or muscular dystrophy. It has huge potential for patients and the industry. ATMPs represent a quickly developing field of interest.Report Overview @ https://www.transparencymarketresearch.com/advanced-therapy-medicinal-products-market.html
Although the vast majority of the products are in an early stage of development, the consolidated trial stage and the possibility to cure serious incessant conditions indicate that ATMPs may achieve the market sooner than standard treatments. Targeted treatments have opened the path for new trial strategies from which ATMPs could profit to get early access. ATMPs are regulated and authorized for marketing by the European Medicines Agency (EMA) in the EU and the Food and Drug Administration (FDA) in the U.S, each of which have specific routes to market depending on the product’s legal categorization.
Based on the type of therapy, advanced therapy medicinal products (ATMPs) are classified as somatic cell therapy medicinal product (SCTMP), tissue engineered medicinal product (TEP), gene therapy medicinal product (GTMP), and combined ATMPs. Cell therapy products like cancer vaccines are growing at a faster rate than gene therapy like Glybera, Strimvelis, and tissue engineered medicinal products due to the number of products advancing in clinical phase development and more number of clinical trials in progress.
Download Report Brochure @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=31298
In addition to regulatory oversight of clinical studies, such as immune-oncology study and technological advancement in genetic engineering tools like talen, RNAi etc. boosts the market of advanced therapy medicinal products. However, a range of issues from a lack of sustainable funding models to insufficient transparency and regulatory guidance for biopharmaceutical companies is still challenging the greater uptake of these innovative treatments.
Geographically, the advanced therapy medicinal products market can be segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America held the dominant share of the global market due to the presence of key players and the advancement in gene and cell therapy with medical support. Moreover, cellular and gene therapy-related research and development in the region continue to grow at a fast rate, with a number of products advancing in clinical development. In addition to regulatory oversight of clinical studies, the Center for Biologics Evaluation and Research provides proactive scientific and regulatory advice to medical researchers and manufacturers in the area of novel product development.
In Europe in EU, regulatory framework for such products was established with the Regulation (EC) 1394/2007, which came into force on December 30, 2008. The regulation established the centralized marketing authorization procedure as the applicable route to authorize advanced therapies. Furthermore, it established a new committee within the European Medicines Agency (EMA), known as the Committee for Advanced Therapies (CAT) which is responsible for assessing the quality, safety, and efficacy of advanced therapies which helps to expand the market in the particular region. However, opportunities for the market are growing in Asia Pacific countries such as India, China, and Japan as the factors driving the market are favorable in these highly populated countries. This is attributed to rapid rise in health care infrastructure and high level of variable diseases like cancer. In the Middle East and African countries, the development of healthcare projects and the economy helps to drive the market for advanced therapy medicinal products.
Pre-Book Full Report @ https://www.transparencymarketresearch.com/checkout.php?rep_id=31298<ype=S
Key players in the advanced therapy medicinal products (ATMPs) market are GSK, Uniqure, Kite Pharma, Pfizer, Adaptimmune, Bluebird Bio Inc., BioMarin Pharmaceutical, Novartis, GE Healthcare, and Shire Biotechnology.
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector - such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports strive to provide clients to serve their overall research requirement.
US Office Contact
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: https://www.transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Advanced Therapy Medicinal Products Market Key Growth Factors and Forecast Upto 2025 here
News-ID: 906612 • Views: …
More Releases from Transparency Market Research

Medium Voltage Fuse Market Outlook 2031: Global Market to Reach US$ 1.8 Billion …
The global medium voltage fuse market is steadily transitioning from a traditional grid-protection niche to a strategic enabler of modern power systems. Rising investments in renewable energy integration, large-scale electrification programs, and infrastructure upgrades are reshaping demand patterns worldwide. Medium voltage fuses-typically rated between 1 kV and 35 kV-are no longer viewed as passive safety components; instead, they are increasingly recognized as critical assets for grid stability, asset protection, and…
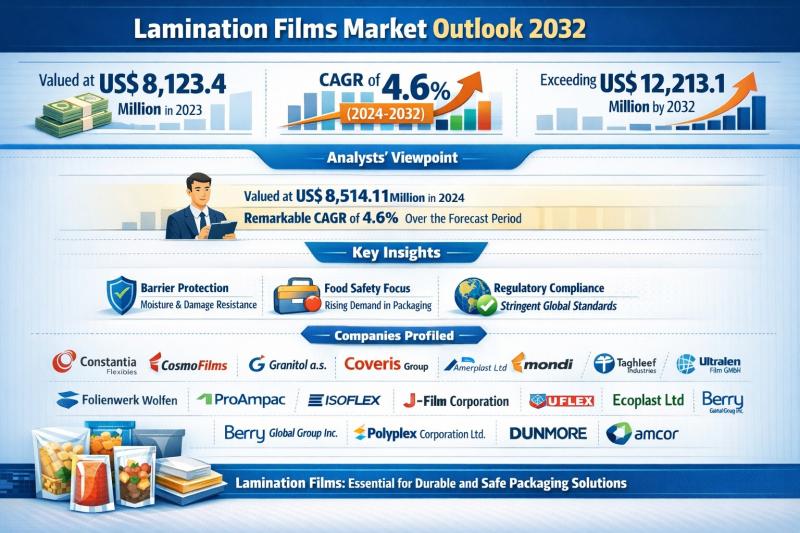
Lamination Films Market Outlook 2032: Global Industry Size to Surpass US$ 12.21 …
The global lamination films market was valued at US$ 8,123.4 million in 2023 and is forecast to reach US$ 8,514.1 million in 2024. Over the forecast period from 2024 to 2032, the market is projected to expand at a compound annual growth rate (CAGR) of 4.6%, ultimately exceeding US$ 12,213.1 million by 2032. This steady growth trajectory reflects the indispensable role of lamination films in modern packaging ecosystems across food,…

Global Curcumin Market Outlook 2031: Natural Antioxidant Demand, Regional Growth …
The global curcumin market is entering a structurally strong growth phase, underpinned by rising consumer preference for natural, plant-based ingredients and increasing clinical validation of curcumin's antioxidant and anti-inflammatory properties. As consumers shift away from synthetic additives and chemical-based therapeutics, curcumin is emerging as a high-value bioactive ingredient across , functional foods, cosmetics, and pharmaceuticals. Premiumization trends in organic products, combined with regulatory validation from food safety authorities, are expected…

Hemoglobin A1c Testing Devices Market to be Worth More Than USD 3.3 Bn by 2034 - …
The global Hemoglobin A1c (HbA1c) Testing Devices Market was valued at US$ 1.4 Bn in 2023 and is projected to expand at a CAGR of 7.7% from 2024 to 2034, reaching more than US$ 3.3 Bn by the end of 2034. The market growth is primarily attributed to the increasing global burden of diabetes, growing awareness about disease management, and technological advancements in diagnostic devices.
Get a concise overview of key…
More Releases for ATMPs
ATMPs Consulting Market Insights on Commercialization Support Early Phase Develo …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMPs Consulting Market- (By Phase (Early-Stage Development, Clinical Development, Commercialization), By Type (Gene therapy medicinal products, Somatic cell medicinal products, Tissue-engineered medicinal products)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global ATMPs Consulting Market is expected to show a CAGR of 8.6% during a forecast…
ATMPs Consulting Market Investments, Share and Revenue Analysis Report to 2034
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMPs Consulting Market- (By Phase (Early-Stage Development, Clinical Development, Commercialization), By Type (Gene therapy medicinal products, Somatic cell medicinal products, Tissue-engineered medicinal products)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global ATMPs Consulting Market is expected to show a CAGR of 8.59% during a forecast…
ATMPs Consulting Market Opportunities and Challenges in Clinical Development and …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMPs Consulting Market- (By Phase (Early-Stage Development, Clinical Development, Commercialization), By Type (Gene therapy medicinal products, Somatic cell medicinal products, Tissue-engineered medicinal products)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global ATMPs Consulting Market is expected to show a CAGR of 8.59% during a forecast…
ATMPs Consulting Market Exclusive Overview Report | 2024-2031
ATMPs Consulting Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMPs Consulting Market- (By Phase (Early-Stage Development, Clinical Development, Commercialization), By Type (Gene therapy medicinal products, Somatic cell medicinal products, Tissue-engineered medicinal products)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce…
ATMPs Consulting Market Latest Trends and Future Aspect Analysis
ATMPs Consulting Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMPs Consulting Market- (By Phase (Early-Stage Development, Clinical Development, Commercialization), By Type (Gene therapy medicinal products, Somatic cell medicinal products, Tissue-engineered medicinal products)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce…
ATMPs Consulting Market Growth and Restrain Factors Analysis 2023-2031
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global ATMPs Consulting Market- (By Phase (Early-Stage Development, Clinical Development, Commercialization), By Type (Gene therapy medicinal products, Somatic cell medicinal products, Tissue-engineered medicinal products)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global ATMPs Consulting Market is expected to show a CAGR of 8.59% during a forecast…
