Press release
Boehringer Ingelheim: Fill & Finish with PAS-X

In state-of-the-art clean rooms which meet the requirements of international authorities, the drugs are filled aseptically into vials or syringes. Source: Boehringer Ingelheim
Lueneburg, Germany, June 16, 2009 - At its Biberach production site in Germany, Boehringer Ingelheim is now carrying out its aseptic fill & finish processes and 100% visual inspection in BioPharma Operations with PAS-X Manufacturing Execution System (MES) software. Boehringer Ingelheim had already been using Werum's MES since 2002 to manufacture biopharmaceutical active ingredients in the largest cell culture plant in Europe. When it started production, this plant also set new technological standards.
Maximum Sterility
Specific criteria and conditions must be considered for fill and finish processes. All relevant information on preceding production stages with a significant effect on the sterility of the finished product must be documented, such as passing airlocks, autoclaving, or decontamination. Defined procedures and expiry dates have to be observed. In state-of-the-art clean rooms, which meet the standards specified by international authorities, the active ingredient batches produced with PAS-X are aseptically filled into vials or syringes and some are freeze-dried.
A number of products are temperature-critical and have a limited expiry date at room temperature.The system must ensure that their expiry dates are not exceeded. When the filling process is complete, all products are required to undergo a 100% visual inspection before they are packaged and released.
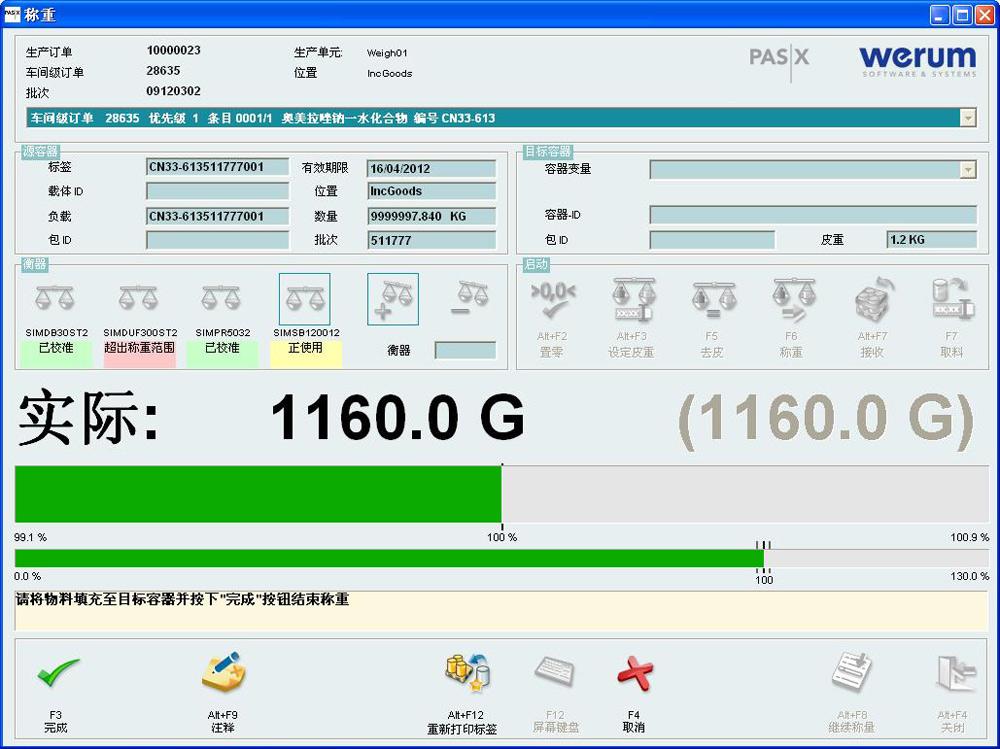
Filling and Visual Inspection with PAS-X
PAS-X has proven its worth in biopharmaceutical active ingredient manufacturing. Now it is also becoming the hub of operative process control in fill & finish and visual inspection operations. The most important MES functions in this area include MASTER BATCH RECORDS MANAGEMENT, which defines all process specifications, and ELECTRONIC BATCH RECORDING, which is responsible for the actual execution processes.
PAS-X ensures that equipment statuses and batch or log information about individual objects are exchanged within the supply chain. In addition, the FINITE SCHEDULING function in PAS-X enables finite planning of processes, and its WAREHOUSE MANAGEMENT function controls all in-company logistics operations.
Integrated System Landscape
PAS-X has made it possible to integrate the fill and finish process seamlessly into the IT landscape of Boehringer Ingelheim. In particular, this integration work involved the ERP system (SAP), the LIMS system and the systems for deviation and document management.
Werum Software & Systems (www.werum.com) is the leading supplier of Manufacturing Execution Systems (MES) for the pharmaceutical and bio-pharmaceutical industries worldwide. Fourteen of the world's top 20 pharmaceutical companies and leading biotech companies use Werum's MES product suite PAS-X to run their manufacturing business. Outstanding PAS-X features are the functions ensuring compliance and boosting performance. Werum's solutions and services range from software consulting, creation of functional specifications, and software development to turn-key delivery of integrated and validated MES. A global partner network ensures reliable local support services. Founded in 1969, the IT company employs 315 people at different locations. Werum is headquartered in Lueneburg (Northern Germany) and has regional offices in St. Augustin and Hausach. Its U.S. subsidiary Werum America is headquartered in Parsippany, New Jersey, and has additional offices in Cary, North Carolina, and in Pasadena, California.
Werum Software & Systems, Tel. USA +1 (973) 644-4000, Tel. Europe +49 (0)4131 8900-0, Press Contact: Volker Mensing, mensing@werum.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Boehringer Ingelheim: Fill & Finish with PAS-X here
News-ID: 84202 • Views: …
More Releases from Werum Software & Systems

13th PAS-X User Group Meeting in Germany
13th PAS-X User Group Meeting in Germany
Werum Manufacturing IT Conference with record attendance
Lueneburg, Germany, 26 October 2011 – The 13th international PAS-X User Group Meeting took place from 13 to 14 October 2011 in Lueneburg, Germany, with a record attendance. On the invitation of Werum Software & Systems AG, almost 200 pharmaceuticals experts from approximately 20 countries exchanged their thoughts and ideas on the use of the PAS-X production management…

Indian pharmaceutical major Dr. Reddy's chooses to implement Werum's MES
Dr. Reddy's Laboratories Ltd. deploys PAS-X at Hyderabad site
Lueneburg, Germany, August 20, 2010 – Dr. Reddy's Laboratories Ltd. has decided to introduce PAS-X, the Manufacturing Execution System (MES) by Werum Software & Systems, at its site in Bachupally, Hyderabad.
Dr. Reddy's plans to optimize the efficiency, quality, compliance and performance of its production processes with PAS-X. Werum is the leading supplier of MES systems for the pharmaceutical and biopharmaceutical industries…

MSD Packaging Lines run with PAS-X
Lueneburg, Germany, August 17, 2010 - MSD deployed PAS-X, the Manufacturing Execution System (MES) by Werum Software & Systems, to run its packaging line operations in Heist-op-den-Berg, Belgium in order to increase the compliance level during packaging operations and subsequently shorten lead times to improve delivery performance. Operations include fully automated blister lines and filling lines, semi-automated packaging lines and manual packaging processes. The project for introducing PAS-X was completed…
Successful rollout of PAS-X MES Weighing & Dispensing at AstraZeneca in India an …
Manufacturing Execution System projects completed successfully
Lueneburg, Germany, March 25, 2010 - Manufacturing Execution System (MES) software provider Werum Software & Systems has successfully finalized the installation of PAS-X MES in AstraZeneca's Wuxi site in the Jiangsu Province, China. This first-time MES project in the Chinese pharmaceutical industry follows an employment of Werum's PAS-X MES software in AstraZeneca's Indian production site in Bengaluru (formerly Bangalore) in 2009.
Both MES projects are part…
More Releases for Boehringer
Anticoagulation Therapy Market Statistical Forecast, Trade Analysis 2024 -Boehri …
DataM Intelligence has published a new research report on "Anticoagulation Therapy Market Size 2024". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Free Sample Research PDF -…
Ticagrelor Market 2027 Top Key Players are AstraZeneca, Eli Lilly, Boehringer, I …
MRFR is the Leading Brand in The Research Company who Recently Published Ticagrelor Market Research Reports which includes Study of growth, Regional Analysis, Top Industry Players Formation, Major Drivers, Upcoming Trends and Forecast to 2027.
Global Ticagrelor Market - Overview
Ticagrelor is an antiplatelet drug which is a member of a section of pharmaceuticals which tackle the problem of platelet aggression. The Global Ticagrelor Market is expected to grow at a healthy…
Heart Attack Preventing Drugs Demand Boosting Ticagrelor Market by 2027 | AstraZ …
The Global Ticagrelor Market is segmented into the various regions including Americas, Europe, Asia Pacific, and the Middle East & Africa. The Americas accounts for the largest market share of about more than ~40% of the market share due to extensive use product by the patients discharged from hospitals in the regions, major manufacturers developing the product in the region.
Europe is the second largest market of Ticagrelor followed by Asia…
Ticagrelor Market 2027 Sketch | By Prominent Players; AstraZeneca, Eli Lilly, Bo …
Ticagrelor is an antiplatelet drug which is a member of a section of pharmaceuticals which tackle the problem of platelet aggression. The global ticagrelor market is expected to grow at a healthy growth rate during the forecast period 2017-2027. The major factors influencing the growth of the market include high patient pool suffering from heart problems, increasing development in the product by the companies, commercialization and improving market access scenarios…
Pharmacovigilance Market Demand Landscape 2025- Accenture, Boehringer Ingelheim
Global Pharmacovigilance Market: Snapshot
Pharmacovigilance (PV) is a process refereeing to the detection, collection, prevention, and monitoring of negative effects that may occur because of the use of pharmaceutical products and other drugs. PV services cater to a wide range of drug related activities such as discovery of a drug to its commercialization. It also helps with the utilization of tools and software that reviews, classifies data on drugs and pharmaceutical…
biocrea and Boehringer Ingelheim Conclude Asset Purchase Agreement
Radebeul, Germany, February 29, 2012 – biocrea today announced the completion of an asset purchase and licensing deal with Boehringer Ingelheim. Under the terms of the agreement, biocrea will receive an undisclosed payment and will transfer the exclusive global rights for certain research programs originating from its phosphodiesterase (PDE) platform to Boehringer Ingelheim. This includes biocrea´s PDE2 inhibitors and its most advanced compound BCA909.
“This transaction marks a key milestone in…