Press release
Pharmacovigilance and Drug Safety Software Market Set for Huge Growth in the Near Future
The global Pharmacovigilance And Drug Safety Software Market is projected to create new opportunities in the coming years on account of the escalating complexity of drug safety regulations. Among the key contributing factors of the market could be tighter regulatory inspections implemented to control the rise of the incidences of drug-associated adverse events and spiraling concerns pertaining to patient safety.Pharmaceutical and biotechnology companies are predicted to largely adopt pharmacovigilance software for curbing the burden on medical outlay and facilitating clinical trial programs. The fortifying growth of contract research organizations (CROs) that perform pharmacovigilance for pharma firms could also strengthen the growth of the global pharmacovigilance and drug safety software market.
Sample With Latest Advancements @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=31112
As per Transparency Market Research (TMR), the global pharmacovigilance and drug safety software market could earn a US$187.0 mn by 2022 end after advancing from a US$143.6 mn achieved in 2017 at a CAGR of 5.4%.
Global Pharmacovigilance and Drug Safety Software Market: Major Insights
The world pharmacovigilance and drug safety software market is envisaged to gain momentum from crucial applications in clinical research for performing post-marketing surveillance roles and conducting clinical studies. On a global platform, some of the most commonly used pharmacovigilance software could be Argus and ArisG. The benefits that such software bring to the table, including decreased redundancy in data and ease of access, could strongly fuel the demand in the market. In the foreseeable future, the usage rate of pharmacovigilance tools is envisioned to receive a good boost due to the rising pressure on drug manufacturers to identify early indications of drug-related adverse interactions and increasing withdrawals of high-profile drugs.
Browse Our Press Releases For More Information @ https://www.transparencymarketresearch.com/pressrelease/pharmacovigilance-drug-safety-software-market.htm
The world pharmacovigilance and drug safety software market is forecasted to be cataloged according to software type, end user, and delivery mode. In terms of segmentation by type of software, fully integrated software, issue tracking software, drug safety audits software, and adverse event reporting software could be prominent markets for pharmacovigilance and drug safety software. The adverse event reporting software market could rise at a 6.2% CAGR.
By end user, the world pharmacovigilance and drug safety software market could see a segmentation into business process outsourcing (BPO) firms, CROs, pharma and biotech companies, and other pharmacovigilance service providers. On the basis of mode of delivery, there could be important segments such as on-premise and cloud-based keeping the hopes alive for growth.
Geographically, North America could secure a top position in the world pharmacovigilance and drug safety software market while raking in a US$79.2 mn by the end of 2022. Between 2017 and 2022, Europe is prophesied to make its contribution to the market by creating an absolute revenue opportunity that could be greater than that expected to be achieved by Asia Pacific except Japan (APEJ) during the forecast period. Markets such as Japan, Latin America, the Middle East and Africa (MEA) could be other key segments of the market.
Browse Our Table of Content @ https://www.transparencymarketresearch.com/report-toc/31112
Global Pharmacovigilance and Drug Safety Software Market: Vendor Landscape
Some of the crucial companies functioning in the worldwide pharmacovigilance and drug safety software market could be UMBRA Global LLC, AB Cube, United BioSource Corporation, Sparta Systems, Inc., Oracle Corporation, EXTEDO GmbH, Ennov Solutions, Inc., and ArisGlobal.
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. We have an experienced team of Analysts, Researchers, and Consultants, who us e proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR Syndicated Research report covers a different sector – such as pharmaceuticals, chemical, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, our syndicated reports thrive to provide clients to serve their overall research requirement.
Contact
Transparency Market Research
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: http://www.transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance and Drug Safety Software Market Set for Huge Growth in the Near Future here
News-ID: 779818 • Views: …
More Releases from Transparency Market Research

Metal Cans Market Valued at USD 71.7 Bn in 2025, Set to Reach USD 100.3 Bn by 20 …
The global Metal Cans Market was valued at US$ 71.7 Bn in 2025 and is projected to reach US$ 100.3 Bn by 2036, expanding at a steady CAGR of 3.1% from 2026 to 2036. The market's growth trajectory is supported by the accelerating transition toward sustainable and infinitely recyclable packaging formats, particularly in the beverage and food industries.
Explore pivotal insights and conclusions from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=706
North America…
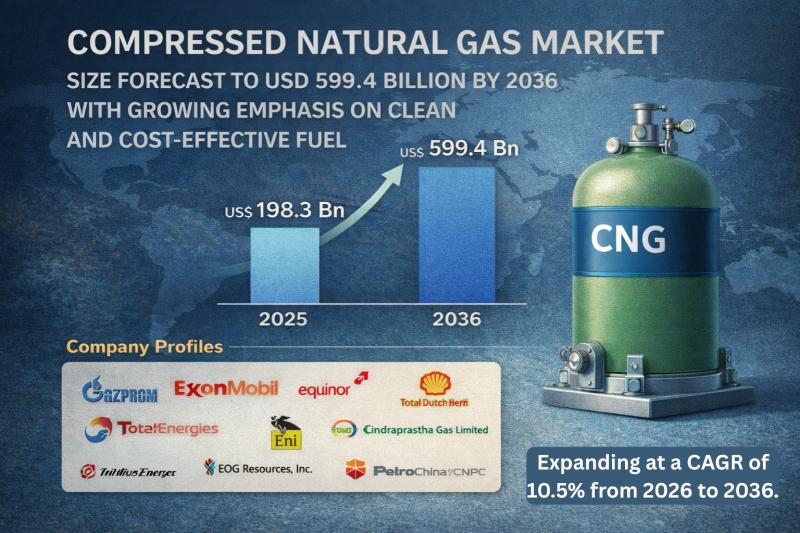
Compressed Natural Gas Market Size Forecast to USD 599.4 Billion by 2036 with Gr …
Compressed Natural Gas Market Outlook 2036
The global compressed natural gas (CNG) market was valued at US$ 198.3 Bn in 2025 and is projected to reach US$ 599.4 Bn by 2036, expanding at a robust CAGR of 10.5% from 2026 to 2036. Market growth is driven by rising demand for cleaner alternative fuels, supportive government policies promoting low-emission transportation, and expanding natural gas infrastructure worldwide.
👉 Get your sample market research report…
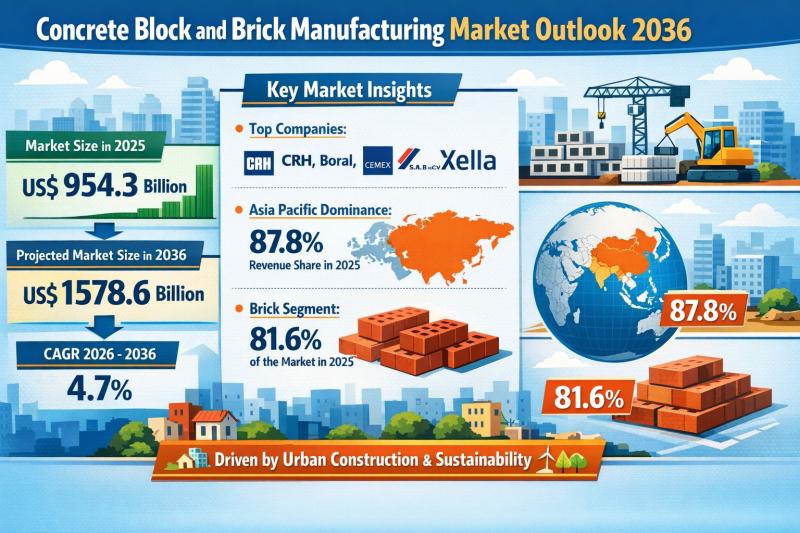
Concrete Block and Brick Manufacturing Market Outlook 2036: Surging Urban Infras …
The global concrete block and brick manufacturing market reached a valuation of US$ 954.3 billion in 2025 and is projected to expand to US$ 1,578.6 billion by 2036, growing at a compound annual growth rate (CAGR) of 4.7% from 2026 to 2036. The steady rise reflects the sustained demand for masonry materials across residential, commercial, and infrastructure projects worldwide.
Historical data from 2021 to 2025 demonstrates consistent volume growth, while forward…
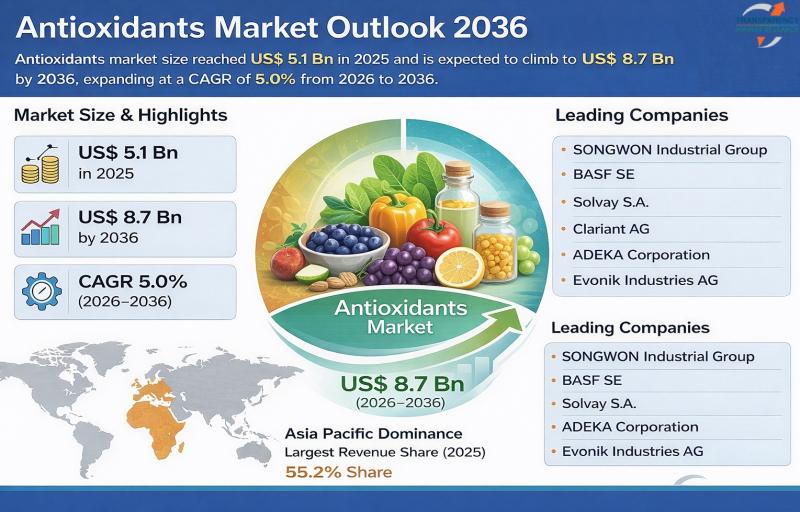
Antioxidants Market to Reach US$ 8.7 Billion by 2036, Fueled by Health Awareness …
The global antioxidants market was valued at US$ 5.1 Billion in 2025 and is projected to reach US$ 8.7 Billion by 2036, expanding at a CAGR of 5.0% from 2026 to 2036. The market is witnessing steady growth driven by increasing health awareness, expanding food preservation applications, rising demand for functional foods, and growing use of antioxidants in cosmetics and pharmaceuticals.
Antioxidants are compounds that inhibit oxidation and neutralize free radicals,…
More Releases for Pharmacovigilance
Top Pharmacovigilance Companies Analysis By 2031
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
Download PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpenPR&utm_medium=10379
The List of Companies
• #Accentures
• Bristol-Myers Squibb Company
• Linical Accelovance
• Cognizant
• Covance Inc.
• F. Hoffmann-La Roche Ltd.
• GlaxoSmithKline plc.
• ICON plc
• Capgemini (IGATE Corporation)
Clinical…
Pharmacovigilance - Scope and Research Methodology
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Pharmacovigilance Market report covers analysis by Clinical Trial Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), Service Provider (In-House and Contract Outsourcing), Type of Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event…
Pharmacovigilance World 2025 Conference & Expo
We are delighted to welcome you to the Pharmacovigilance World 2025, and we are confident that your active participation will contribute to the advancement of drug safety practices. Together, let us strive towards a safer and more vigilant healthcare system that prioritizes patient well-being and ensures the continued benefit of medications worldwide.
As medical science advances, so does our understanding of drug safety and the need for vigilance when it comes…
Top Factor Driving Pharmacovigilance Market Growth in 2025: Research And Develop …
How Are the key drivers contributing to the expansion of the pharmacovigilance market?
The escalation in research and development undertakings stimulates growth in the pharmacovigilance market. Pharmaceutical organizations can create novel and superior drugs through enhanced safety profiles by allocating resources to R&D. The intensive testing in preclinical and clinical stages during the drug development protocol allows early recognition of potential safety issues, paving the way for adequate risk reduction approaches.…
Monitoring Medication Safety with Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Pharmacovigilance plays a significant role in pharmaceutical and biotechnological sectors in designing of drugs and their interactions. The pharmacovigilance involves collecting information from healthcare providers and patients to know about the hazards associated with medications.
Download Sample PDF at: https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpnePR&utm_medium=10776
Increasing cases of adverse drug reactions…
Pharmacovigilance Market Opportunity Analysis by 2028
Pharmacovigilance Market: Introduction
According to the report, the global pharmacovigilance market was valued at US$ 6.1 Bn in 2020 and is projected to expand at a CAGR of 8.8% from 2021 to 2028. Pharmacovigilance activities are defined as science used for detection, assessment, understanding, and prevention of adverse effects of drugs and vaccines. Drugs and vaccines go through rigorous testing in the clinical trials to check their safety and efficacy before…
