Press release
Interesting Research Report on the Future of Clinical Trials Market
Clinical trials are research studies performed on humans to gain specific information about biomedical interventions such as novel vaccines, devices, treatments and drugs and thereby generating safety data. Clinical trials are regulated by health authorities and ethics committees.Documents required for performing clinical trials are investigator’s brochure (IB) which include current and relevant scientific information about the investigational product, United States Food and Drug (FDA) form 1572, protocol and amendments, inform consent, other written information for participants, recruitment advertisement, financial disclosure form (FDF), master clinical trial agreement (MCTA), institutional review board (IRB) approval, medical licensure, training records, laboratory accreditation, visit monitor reports, miscellaneous document, signature sheet and documentation of investigational drug destruction. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) brings together regulatory authorities of Europe, the United States, Japan and experts from pharmaceutical industry to frame and regulate the technical and scientific aspects of pharmaceutical product registration. The Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) states rules and standard guidelines for clinical trials. ICH guidelines are followed as law by several countries in the world.
Request Report Sample@ http://www.futuremarketinsights.com/reports/sample/rep-gb-384
Clinical trials are conducted in four phases namely, Phase I, II, III and IV. Phase I is conducted for safety, phase II is conducted for efficacy, phase III is conducted for final confirmation of safety and efficacy and phase IV is conducted for post sales studies. Risk to participants involved in clinical trials decreases from phase I to phase VI. Number of participants increases from phase I to phase IV resulting in increasing cost of trials. Based on the phases of clinical trials, global clinical trials market is segmented as follows:
Phase I
Phase II
Phase III
Phase IV
Based on indication, global clinical trials market is classified as follows:
Blood disorders
Ophthalmology
Autoimmune diseases
Circulatory diseases
Cancer
Genitourinary diseases
Congenital diseases
Musculoskeletal diseases
Central nervous system (CNS)
Infections
Dermatology
Metabolic disorders
Cardio vascular system (CVS) diseases
Gastrointestinal diseases
Mental disorders
Others
Visit For TOC@ http://www.futuremarketinsights.com/toc/rep-gb-384
Being relatively costly process, in order to reduce economic burden on company and shift focus on core business activities, many companies outsource their clinical trial activities to contract research organizations (CROs). Contract research organizations provide services such as clinical trial management, clinical research and preclinical research. Factors such as advancement in technology and increasing demand of innovative solutions in healthcare industry are driving the market of global clinical trials towards growth. On the other hand, factors such as high cost and stringent regulations are restraining the growth of clinical trials market globally. Geographically, the global clinical trials market is segmented into North America, Europe, Asia-Pacific and Rest of the World.
North America is the leading consumer of global clinical trials solutions, followed by Europe. Ample availability of funds to outsource clinical trials serves as the major growth driver for the North America clinical trials market. Asia-Pacific demonstrates impressive growth potential for clinical trials market and is expected to show the highest growth rate as compared to other regions in the world. Countries such as India are attractive markets due to advantages such as availability of skilled practitioners and availability government support in terms development of outsourcing hubs thus attracting pharmaceutical and biotechnology companies to outsource clinical trial activities to CROs in this region. Some of the market leaders contributing to the global clinical trials market include Eli Lilly and Company, Novo Nordisk A/S, Ranbaxy Laboratories, Ltd., Sanofi Aventis A.S. and Roche Group.
ABOUT US:
Future Market Insights (FMI) is a leading market intelligence and consulting firm. We deliver syndicated research reports, custom research reports and consulting services, which are personalized in nature. FMI delivers a complete packaged solution, which combines current market intelligence, statistical anecdotes, technology inputs, valuable growth insights, an aerial view of the competitive framework, and future market trends.
CONTACT:
Future Market Insights
616 Corporate Way, Suite 2-9018,
Valley Cottage, NY 10989,
United States
T: +1-347-918-3531
F: +1-845-579-5705
Email: sales@futuremarketinsights.com
Website: www.futuremarketinsights.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Interesting Research Report on the Future of Clinical Trials Market here
News-ID: 668738 • Views: …
More Releases from Future Market Insights
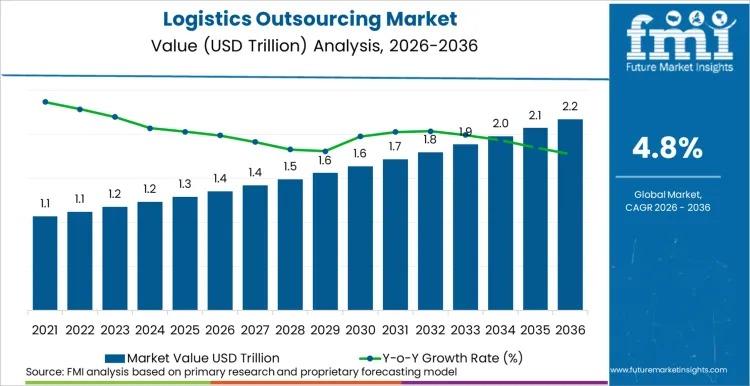
Global Logistics Outsourcing Market to Reach USD 2.2 Trillion by 2036 at 4.8% CA …
The global Logistics Outsourcing Market is projected to expand from USD 1.4 trillion in 2026 to USD 2.2 trillion by 2036, registering a CAGR of 4.8% during the forecast period. According to Future Market Insights (FMI), enterprises are accelerating outsourcing strategies to enhance supply chain resilience, digital transparency, and operational flexibility in an increasingly volatile global trade environment.
Demand dynamics are heavily influenced by the need for end-to-end visibility, omnichannel fulfillment…
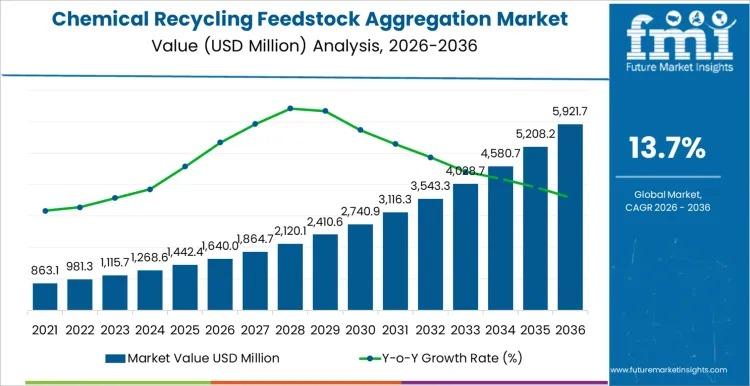
Chemical Recycling Feedstock Aggregation Market to Reach USD 5,921.7 million by …
The global Chemical Recycling Feedstock Aggregation Market is projected to grow from USD 1,640.0 million in 2026 to USD 5,921.7 million by 2036, registering a CAGR of 13.7%. The expansion reflects structural scaling of chemical recycling plants that depend on consistent, specification-aligned plastic waste streams rather than fragmented sourcing models.
As pyrolysis and depolymerization capacities expand worldwide, aggregators are investing in centralized hubs that integrate pre-sorting, blending, contamination control, and logistics…
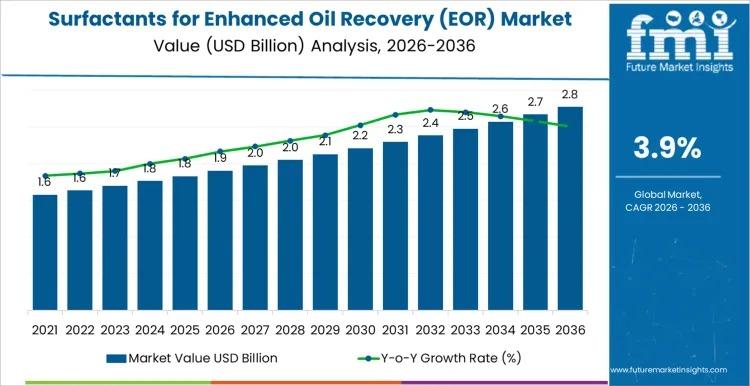
Global Surfactants for Enhanced Oil Recovery Market to Reach USD 2.9 Billion by …
The global Surfactants for Enhanced Oil Recovery (EOR) Market is projected to grow from USD 1.9 billion in 2026 to USD 2.9 billion by 2036, registering a CAGR of 3.85%. Market expansion is closely tied to mature oilfield economics, where incremental recovery gains justify chemical investment. Rather than broad upstream expansion, growth is concentrated in technically validated, project-specific deployments.
Long evaluation timelines, pilot testing requirements, and reservoir heterogeneity slow rapid scale-up,…
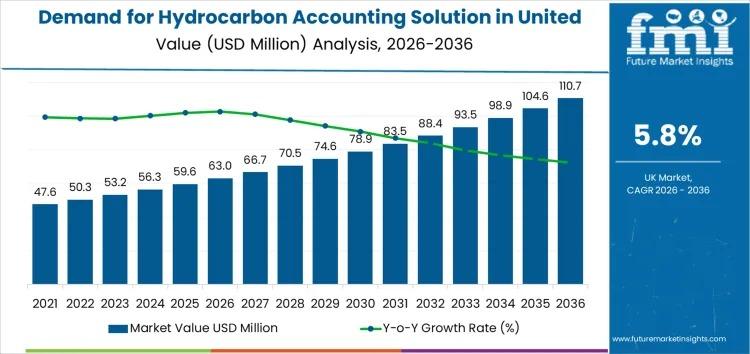
UK Hydrocarbon Accounting Solution Market to Reach USD 110.7 Mn by 2036 at 5.8% …
The Demand for Hydrocarbon Accounting Solution in United Kingdom is projected to expand from USD 63.0 million in 2026 to USD 110.7 million by 2036, registering a CAGR of 5.8% over the forecast period. The market's growth trajectory reflects accelerating regulatory oversight, digital oilfield deployment, and enterprise-level data governance initiatives across upstream and midstream operations.
As operators confront tightening volumetric reporting standards and emissions accountability frameworks, hydrocarbon accounting solutions are becoming…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
