Press release
Pharmacovigilance Market - Positive long-term growth outlook
Pharmacovigilance is a key component of an effective drug regulation system for monitoring and evaluating adverse drug reactions (ADRs). Pharmacovigilance activities are an important part of clinical research and are growing at a significant pace. At present, the global network of pharmacovigilance centers, harmonized by Uppsala Monitoring Centre, are operating on the global level for appropriate functioning of the process of drug safety monitoring across the world. Large volume of international ADR reports collected in a central database would serve as a contributing factor to the effort of national drug regulatory authorities, thus improving the safety profile of drugs that would help avoid drug related disasters. In addition, growing public health awareness and expectation in relation to the safe use of medicines and medical interventions are some of the major factors contributing to the growth of the global pharmacovigilance market. Furthermore, increasing number of national pharmacovigilance centers globally is playing a definitive role in augmenting public awareness about drug safety, thereby supporting the growth of the pharmacovigilance market in the next few years. However, the pharmacovigilance market is facing several hurdles and challenges to develop a better health care system. Challenges that are acting as major restraining factors for pharmacovigilance market are web-based drug information and sales, perceptions to harm and benefit, high risk associated with data security and unavailability of skilled professionals.Based on phases of drug development, the global pharmacovigilance market has been segmented into preclinical studies, clinical trial phase I, II, III and IV or post marketing surveillance. The phase IV or post marketing surveillance segment accounted for the largest share of the pharmacovigilance market in terms of revenue in 2013. Rise in the number of safety concerns pertaining to the marketed product, increasing need for developing systems for comparing safety profiles of similar pharmaceutical products and growth in public health awareness campaigns regarding drug safety issues among the people are the major factors attributed to the high growth of the segment. In addition, the clinical trial phase III segment is expected to grow at the highest CAGR due to the rising need for drug safety monitoring and evaluation in phase III before the drug manufacturer can apply for market authorization application. Additionally, phase III clinical trial focuses on drug safety and efficacy in diverse sub-groups, wherein the risk-benefit ratio is developed, monitored and updated accordingly. Biopharmaceutical companies are in the process of developing advanced clinical trial phases that would be more specific to drug safety. Rigorous pharmacovigilance activities are expected to be made compulsory during the commencement of various clinical trial phases that would be helpful in actively managing high-risk medicines.
This 151 page report gives readers a comprehensive overview of the Pharmacovigilance Market. Browse through 5 data tables and 46 figures to unlock the hidden opportunities in this market: http://www.transparencymarketresearch.com/pharmacovigilance-market.html
Based on methods of performing pharmacovigilance, the market has been categorized into spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring, and EHR mining. Spontaneous reporting method was the largest segment in terms of revenue in 2013. Factors attributed to the growth of the segment are easy simulation of realistic datasets that provides better drug evaluation and comparison, and better assessment of automatic signal generation methods proposed within pharmacovigilance. The segment is also expected to maintain its lead during the forecast period from 2014 to 2020, as in this method, healthcare professionals are encouraged to report adverse reactions after which preventive measures such as adding warnings to the product labeling or recalling the product are taken. Moreover, spontaneous reporting is the most appropriate method of detecting new ADRs and generates safety signals that require further examination.
Based on types of services, the pharmacovigilance market has been segmented into in-house and contract outsourcing. The contract outsourcing service segment accounted for the largest share in 2013. The segment is estimated to grow at the highest CAGR during the forecast period. The major factor driving the growth of the segment is the increasing need of pharmaceutical companies to shift focus from non-core to core business activities by outsourcing their pharmacovigilance services. Moreover, outsourcing of pharmacovigilance services is aimed at minimizing operational cost and financial loss incurred from product approval delays or recalls, thereby propelling the growth of the contract outsourcing service segment.
Get accurate market forecast and analysis on the Pharmacovigilance Market. Request a sample to stay abreast on the key trends impacting this market: http://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=1729
Geographically, North America accounted for the largest share of the global pharmacovigilance market in 2013. Factors attributed to the growth of the pharmacovigilance market in North America are increasing mortality rate due to ADRs and escalating patient concerns regarding the safety and efficacy of pharmaceutical products. According to the Centers for Disease Control and Prevention (CDC), ADRs account for more than 100,000 deaths annually and are among the top 10 leading causes of death in the U.S. Moreover, cost benefit obtained by shifting high costs of in-house pharmacovigilance activities to CROs is boosting the growth of the market in North America. However, Asia Pacific is anticipated to witness the highest growth during the forecast period. Factors attributed to the high growth are increased demand for stringent health care regulations in Asia, presence of large patient pool and increasing volume of clinical trials being conducted in this region. According to an article published in the National Center for Biotechnology Information in 2014, serious ADRs are witnessed in 6.7% of patients in India. Additionally, demand for effective pharmacovigilance services and drug safety in increasing in the region due to large number of clinical trials and clinical research activities being carried out in various countries in Asia.
The global pharmacovigilance market is fragmented due to the presence of numerous established as well as emerging organizations. The top companies operating in the pharmacovigilance market are Cognizant Technology Solutions, Accenture plc, Bristol-Myers Squibb, Covance, Inc., Clinquest Group B.V., ICON plc, F. Hoffmann-La Roche Ltd., inVentiv Health, Inc., GlaxoSmithKline plc, iGATE Corporation, Novartis International AG, Wipro Limited, Quintiles Transnational Holdings, Inc., Synowledge LLC, Pharmaceutical Product Development, LLC, (PPD), Sanofi, PAREXEL International Corporation, Pfizer, Inc., and iMEDGlobal Corporation.
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector - such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports strive to provide clients to serve their overall research requirement.
Contact us:
Transparency Market Research
90 State Street,
Suite 700,
Albany
NY - 12207
United States
Tel: +1-518-618-1030
USA - Canada Toll Free 866-552-3453
Email: sales@transparencymarketresearch.com
Website: http://www.transparencymarketresearch.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance Market - Positive long-term growth outlook here
News-ID: 525327 • Views: …
More Releases from Transparency Market Research

Overhead Cranes Market Trends and Forecast 2034: Smart Lifting Solutions, Region …
The global overhead cranes market is entering a steady and technology-driven growth phase, supported by rising industrialization, infrastructure investments, and automation-led transformation in material handling. Manufacturing expansion in developing economies, coupled with large-scale construction of roads, bridges, ports, and industrial facilities, continues to generate sustained demand for overhead cranes. At the same time, the integration of IoT, smart sensors, and automated controls is redefining crane performance standards by improving safety,…
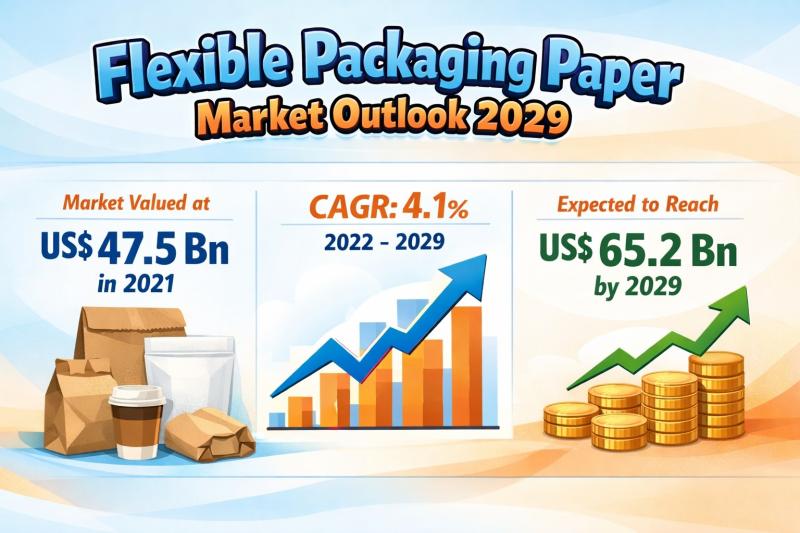
Flexible Packaging Paper Market to be Worth USD 65.2 Bn by 2029 - By Kraft, Glas …
The global Flexible Packaging Paper Market was valued at US$ 47.5 Bn in 2021 and is projected to reach US$ 65.2 Bn by the end of 2029, expanding at a compound annual growth rate (CAGR) of 4.1% from 2022 to 2029. The steady expansion of this market reflects the increasing global preference for sustainable, lightweight, and cost-efficient packaging solutions across multiple industries. The growing shift away from rigid packaging toward…

Insect Repellent Apparel Market Outlook 2034: Rising Demand for Functional, Prot …
The global insect repellent apparel market was valued at US$ 812.2 million in 2023 and is projected to reach US$ 1.6 billion by the end of 2034, growing at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2034. The market is witnessing steady expansion due to increasing awareness of vector-borne diseases, rising outdoor recreational activities, growing adoption of functional textiles, and continuous innovation in fabric treatment technologies.
Insect…
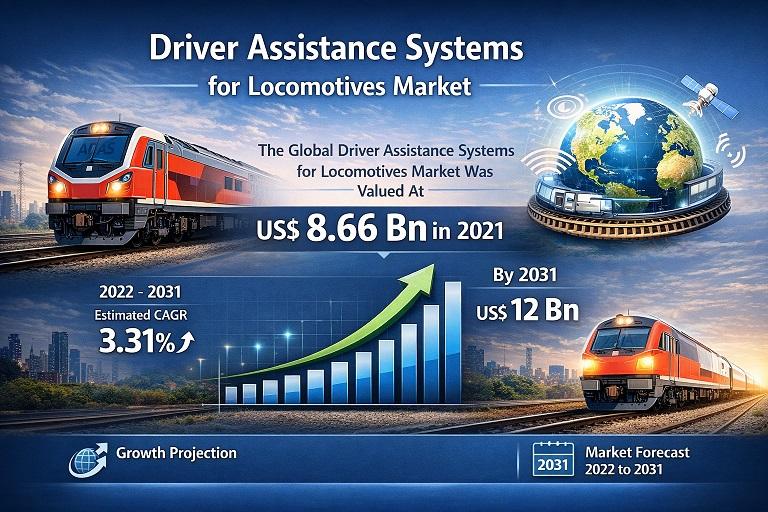
Driver Assistance Systems for Locomotives Market Forecast to Reach USD 12 Bn by …
The Driver Assistance Systems for Locomotives Market is gaining substantial traction as the global railway industry shifts toward enhanced safety, digitalization, and semi-autonomous operations. Rail transport remains one of the most efficient and sustainable modes of transportation for both passengers and freight. However, increasing traffic density, expanding rail networks, and the need to reduce operational risks are driving the demand for advanced driver support technologies.
Explore the Sample Report - Find…
More Releases for Pharmacovigilance
Top Pharmacovigilance Companies Analysis By 2031
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
Download PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpenPR&utm_medium=10379
The List of Companies
• #Accentures
• Bristol-Myers Squibb Company
• Linical Accelovance
• Cognizant
• Covance Inc.
• F. Hoffmann-La Roche Ltd.
• GlaxoSmithKline plc.
• ICON plc
• Capgemini (IGATE Corporation)
Clinical…
Pharmacovigilance - Scope and Research Methodology
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Pharmacovigilance Market report covers analysis by Clinical Trial Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), Service Provider (In-House and Contract Outsourcing), Type of Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event…
Pharmacovigilance World 2025 Conference & Expo
We are delighted to welcome you to the Pharmacovigilance World 2025, and we are confident that your active participation will contribute to the advancement of drug safety practices. Together, let us strive towards a safer and more vigilant healthcare system that prioritizes patient well-being and ensures the continued benefit of medications worldwide.
As medical science advances, so does our understanding of drug safety and the need for vigilance when it comes…
Top Factor Driving Pharmacovigilance Market Growth in 2025: Research And Develop …
How Are the key drivers contributing to the expansion of the pharmacovigilance market?
The escalation in research and development undertakings stimulates growth in the pharmacovigilance market. Pharmaceutical organizations can create novel and superior drugs through enhanced safety profiles by allocating resources to R&D. The intensive testing in preclinical and clinical stages during the drug development protocol allows early recognition of potential safety issues, paving the way for adequate risk reduction approaches.…
Monitoring Medication Safety with Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Pharmacovigilance plays a significant role in pharmaceutical and biotechnological sectors in designing of drugs and their interactions. The pharmacovigilance involves collecting information from healthcare providers and patients to know about the hazards associated with medications.
Download Sample PDF at: https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpnePR&utm_medium=10776
Increasing cases of adverse drug reactions…
Pharmacovigilance Market Opportunity Analysis by 2028
Pharmacovigilance Market: Introduction
According to the report, the global pharmacovigilance market was valued at US$ 6.1 Bn in 2020 and is projected to expand at a CAGR of 8.8% from 2021 to 2028. Pharmacovigilance activities are defined as science used for detection, assessment, understanding, and prevention of adverse effects of drugs and vaccines. Drugs and vaccines go through rigorous testing in the clinical trials to check their safety and efficacy before…
