Press release
Hepatitis E Diagnostic Tests Market: Latest Trends & Insights 2024
Global Hepatitis E Diagnostic Tests Market: OverviewHepatitis E is a liver disease that is enterically transmitted and is caused by hepatitis E virus (HEV). It is one of the five known human hepatitis viruses (A, B, C, D, and E). Hepatitis E spreads through fecal-oral transmission due to fecal contamination of drinking water. The other routes of transmission of hepatitis E virus include transfusion of infected blood products, vertical transmission from a pregnant woman to her fetus, and ingestion of undercooked meat or meat products derived from infected animals. Infections with hepatitis E virus are commonly observed in regions with poor sanitation, including the Middle East, Asia, Central America, and Africa. The epidemiology and serology of HEV differs in developing and developed countries. It is considered to be rare in the U.S. and other developed regions.
Download Exclusive Brochure of This Report :
http://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=16859
Cases of hepatitis E are not clearly distinguishable from other types of viral hepatitis. Hence, diagnosis can be confirmed by testing the presence of RNA and antibodies against HEV. Testing for the presence of IgM and IgG is considered to be indirect tests and testing for the presence of HEV RNA is considered to be direct test. Presence of IgM is the primary test to identify acute infections in patients, which is confirmed by testing for the presence of RNA. Presence of IgG antibodies indicates that the patient has been exposed previously to hepatitis E virus.
This research is a combination of primary and secondary research, conducted for understanding and arriving at trends, used to forecast the expected revenue of hepatitis E diagnostic kits in the near future. Primary research formed the bulk of our research efforts with information collected from in-depth interviews and discussions with a number of key industry experts and opinion leaders. Secondary research involved study of company websites, annual reports, press releases, investor presentations, analyst presentation and various international and national databases.
Global Hepatitis E Diagnostic Tests Market: Scope of the Study
The report provides estimated market size in terms of US$ Mn for each test type, end user, and geography for the period 2014 to 2024, considering the macro and micro environmental factors. The revenue generated from each test type was calculated by considering sero-prevalence rate of hepatitis E, average selling price of the kits, trends in industry, end user trend, and adoption rate across all the geographies.
The market report comprises an elaborated executive summary, which includes market snapshot that provides information about various segments of the market. It also provides information and data analysis of the market with respect to market segments based on test type, end-user, and geography. The market overview section of the report analyzes market dynamics such as drivers, restraints and opportunities that influences the hepatitis E diagnostic tests market in the current and future scenario. The report also provides value chain analysis of the market that describes the sequence of activities involved from identification of the market need to their final reach to the end users.
Market share analysis among the market players is analyzed to signify the contribution of these players in the market in terms of percentage share. All these factors will help the market players to decide about the business strategies and plans to strengthen their positions in the global market. Based on geography, the market has been analyzed for major regions: North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. The study also covers detailed country analysis contributing majorly in the hepatitis E diagnostic tests market.
Companies Mentioned in this report
The report also profiles the major players in the market and provides various attributes such as company overview, financial overview, product portfolio, business strategies, and recent developments. Major companies profiled in the hepatitis E diagnostic tests market report are altona Diagnostics GmbH, Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., Biokit S.A., Dia.Pro - Diagnostic Bioprobes s.r.l, F. Hoffmann-La Roche Ltd., Fast-track diagnostics Ltd., Fortress Diagnostics Limited, Mikrogen GmBH, MP Biomedicals, LLC., and Primerdesign Ltd., among others.
Browse Global Strategic Business Report:
http://www.transparencymarketresearch.com/hepatitis-e-diagnostic-tests-market.html
The Hepatitis E Diagnostic Tests Market has been segmented as follows:
By Test Type
ELISA HEV IgM Test kits
ELISA HEV IgG Test kits
RT-PCR Test kits
Others
By End User
Hospitals
Research Centers
Diagnostic Laboratories
Point of Care
By Geography
North America
U.S.
Canada
Europe
U.K.
Germany
Spain
France
Italy
Rest of Europe
Asia Pacific
China
Japan
India
Australia
Rest of Asia Pacific
Latin America
Brazil
Mexico
Rest of Latin America
Middle East and Africa
A.E.
South Africa
Rest of Middle East and Africa
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector – such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports strive to provide clients to serve their overall research requirement.
US Office Contact
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: www.transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Hepatitis E Diagnostic Tests Market: Latest Trends & Insights 2024 here
News-ID: 467967 • Views: …
More Releases from Transparency Market Research - Pharmaceutical
Neurovascular Devices Market Business Opportunities and Growth Challenges Report
Global Neurovascular Devices Market: Overview
The global population is aging rapidly. According to WHO, the chances of health risks are greater in geriatric people than the young generation. Old age is considered to be one of the biggest risk factors that are responsible for developing various diseases such as cardiovascular and neurological conditions. It is because of these factors, the global neurovascular devices market is experiencing robust growth in the…
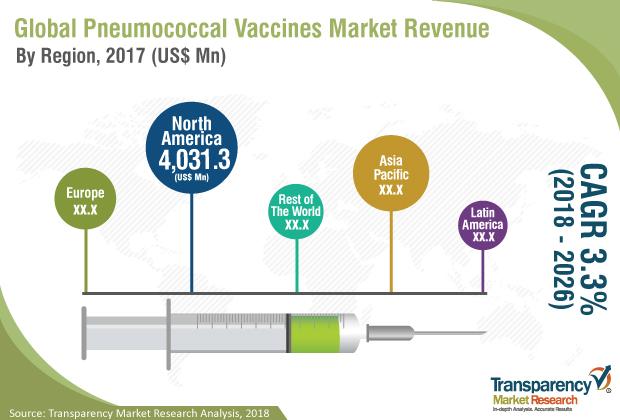
Pneumococcal Vaccines Market anticipated expand at a CAGR of 3.3% from 2018 to 2 …
Transparency Market Research (TMR) has published a new report titled, “Pneumococcal Vaccines Market - Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2018–2026”. According to the report, the global pneumococcal vaccines market was valued at US$ 7,247.6 Mn in 2017 and is anticipated expand at a CAGR of 3.3% from 2018 to 2026. Increase in patient pool, growth of the pharmaceutical industry, government initiatives to increase vaccination programs, rise…
Swab and Viral Transport Medium Market projected to expand at a CAGR of ~3% from …
Swab and Viral Transport Medium Market:
Introduction
Transparency Market Research has published a new report titled, ‘Global Swab and Viral Transport Medium Market ’. According to the report, the global swab and viral transport medium market was valued at US$ 0.9 Bn in 2019 and is projected to expand at a CAGR of ~3% from 2020 to 2030. Viral transport medium (VTM) enables safe transfer of viruses, chlamydia, and mycoplasma for…
Radiofrequency Ablation Devices Market by Product, Geography and Forecast to 202 …
Transparency Market Research (TMR) has published a new report titled, 'Radiofrequency Ablation Devices for Pain Management Market - Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2019-2027'. According to the report, the global radiofrequency ablation devices for pain management market was valued at US$ 543.0 Mn in 2018 and is projected to expand at a CAGR of above 11.0% from 2019 to 2027.
Overview
Radiofrequency ablation is a minimally invasive surgical…
More Releases for Dia
Octalsoft to Attend DIA 2025 Global Annual Meeting in Washington, DC
Herndon, VA - 12 June 2025 - Octalsoft is proud to announce its participation in the DIA 2025 Global Annual Meeting, taking place from June 15 to 19 in Washington, DC. This prestigious event brings together leading voices across regulatory, clinical, safety, and operational domains to address shared industry challenges and co-create the future of clinical research.
Representing Octalsoft at this year's gathering will be our Managing Director, Hiren Thakkar, who…
Welldon's Win At The Design Intelligence Award (DIA)
We are thrilled to announce that Welldon has been honored with the Design Intelligence Award (DIA), a highly prestigious international design competition.
Welldon's WD040 child safety seat stood out from over 8,000 entries, showcasing our innovation and commitment to child safety.
Image: https://ecdn6.globalso.com/upload/p/873/image_product/2024-10/1-1.jpg
What is the DIA?
The Design Intelligence Award (DIA) is China's first international academic award in the field of innovation design, launched in 2014. Supported by the Zhejiang Provincial Government and…
Food and Grocery Retailing Market 2022 Key Consumer Trends by Carrefour, Extra, …
"Food & grocery Retailing in Brazil, Market Shares, Summary and Forecasts to 2022", provides data for historic and forecast retail sales, and also includes information on the business environment and country risk related to Brazil retail environment. In addition, it analyzes the key consumer trends influencing Brazil food & grocery industry.
Food & grocery is the largest sector in the Brazilian retail industry, accounting for 53.9% of total retail sales. The…
Art Gallery Announces Dia Spriggs in Artist Showcase
Jupiter, FL, USA (October 19, 2013) -- Light Space & Time Online Art Gallery is very pleased to announce that wildlife artist Dia Spriggs is the gallery's newest featured artist. Her artworks will now be featured and promoted in gallery's Artist Showcase for the next 30 days.
Dia Spriggs is a self-taught wildlife artist living and creating her art in Florida. She lives with 13 cats, 2 dogs and 2 parrots,…
Meet MakroCare at 16th DIA CDM Japan Annual Workshop
DIA is conducting “16th Annual workshop in Japan for Clinical Data Management” conference in Tokyo, Japan from Feb 7-8, 2013 . Objectives of this workshop are to improve the quality of clinical research and data management activities. This annual workshop will provide information to attendees about the international clinical data management and attendees will get opportunities to network with colleagues in global Clinical Data Management. This workshop is expected…
MakroCare Launches EDC Application in Japanese Version at 15th DIA CDM Conferenc …
DDi (Drug Development informatics), a division of MakroCare, provides clinical products and technology solutions to the pharmaceutical, biotechnology and medical device companies.
DDi is releasing new Japanese edition “mEDC v2.0” in 15th DIA CDM Workshop in Japan. “mEDC v2.0” is a highly comprehensive configurable web-based electronic data capture application used for managing single or multi-site clinical research studies with complete audit trail.
Apart from mEDC, DDi brings other suite of applications specifically…
