Press release
Metastatic Hormone Refractory Prostate Cancer Clinical Trial Pipeline Expands as 180+ Companies Driving Innovation in the Therapeutics | DelveInsight
DelveInsight's, "Metastatic Hormone Refractory Prostate Cancer Pipeline Insight 2025" report provides comprehensive insights about 180+ companies and 200+ pipeline drugs in Metastatic Hormone Refractory Prostate Cancer pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Explore our latest breakthroughs in Metastatic Hormone Refractory Prostate Cancer Research. Learn more about our innovative pipeline today! @ https://www.delveinsight.com/sample-request/metastatic-hormone-refractory-prostate-cancer-pipeline-insight [https://www.delveinsight.com/sample-request/metastatic-hormone-refractory-prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Metastatic Hormone Refractory Prostate Cancer Pipeline Report
* On February 12, 2026- Amgen announced a phase 3, Open-label, Multicenter, Randomized Study of Xaluritamig Plus Abiraterone Versus Investigator's Choice in Participants With Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer
* On February 11, 2026- Kyntra Bio. initiated a study is to evaluate the safety, efficacy, tolerability, and pharmacokinetics (PK) of FG-3246, a cluster of differentiation 46 (CD46) targeting antibody-drug conjugate (ADC), in the treatment of participants with mCRPC who have progressed following treatment with one prior second-generation androgen receptor signaling inhibitor (ARSI) in any setting and no prior taxane therapy in the mCRPC setting.
* On February 09, 2026- Fusion Pharmaceuticals Inc. announced a study is an open-label, multicenter study designed to investigate the efficacy, safety and tolerability of FPI-2265 (225Ac-PSMA-I&T) in combination with Olaparib in participants with mCRPC. The dose optimization Phase 2 part will be investigating the safety, tolerability, and anti-tumor activity of novel dosing regimens of FPI-2265 and Olaparib in participants with metastatic castration-resistant prostate cancer.
* On February 09, 2026- Novartis Pharmaceuticals conducted a Phase I/II, open-label, global, multicenter study assessing the safety and efficacy of the combination of tulmimetostat (DZR123) and JSB462 (luxdegalutamide) versus standard of care in participants with progressive metastatic castrate resistant prostate cancer (mCRPC).
* DelveInsight's Metastatic Hormone Refractory Prostate Cancer Pipeline report depicts a robust space with 180+ active players working to develop 200+ pipeline therapies for Metastatic Hormone Refractory Prostate Cancer treatment.
* The leading Metastatic Hormone Refractory Prostate Cancer Companies such as Lantheus, Merck, Exelixis, Zenith Epigenetics, CellCentric, Karyopharm Therapeutics, Janux Therapeutics, Pfizer, Tavanta Therapeutics, Telix Pharmaceuticals, Jiangsu HengRui Medicine, SOTIO, Antev Ltd., Syntrix Pharmaceuticals, Regeneron Pharmaceuticals, Madison Vaccines, Phosplatin Therapeutics, MacroGenics, RedHill Biopharma, Xencor and others.
* Promising Metastatic Hormone Refractory Prostate Cancer Therapies such as Enzalutamide, Patupilone, Prednisone, Docetaxel, Zactima (vandetanib), MDX-010, Denosumab, RAD001 , and others.
Download for updates and be a part of the revolution in Oncology Care @ Metastatic Hormone Refractory Prostate Cancer Clinical Trials Assessment [https://www.delveinsight.com/sample-request/metastatic-hormone-refractory-prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
The Metastatic Hormone Refractory Prostate Cancer Pipeline Report provides a disease overview, pipeline scenario and therapeutic assessment of the key pipeline therapies in this domain. The Metastatic Hormone Refractory Prostate Cancer Pipeline Report also highlights the unmet needs with respect to Metastatic Hormone Refractory Prostate Cancer.
Metastatic Hormone Refractory Prostate Cancer Overview
Metastatic Hormone-Refractory Prostate Cancer (mHRPC), also known as metastatic castration-resistant prostate cancer (mCRPC), is an advanced stage of prostate cancer in which the disease continues to progress despite the suppression of testosterone to castrate levels. Initially, prostate cancer growth is typically driven by androgens, and hormone therapy is effective in slowing or stopping the disease. However, in mHRPC, cancer cells adapt and continue to grow even in the low-androgen environment. The cancer has also spread beyond the prostate gland to distant sites, most commonly bones and lymph nodes. This stage is associated with more aggressive behavior, increased symptom burden, and limited treatment options, requiring systemic therapies aimed at prolonging survival and improving quality of life.
Metastatic Hormone Refractory Prostate Cancer Emerging Drugs Profile
* PNT2002: Lantheus
A radioconjugate composed of PNT2002, a human prostate-specific membrane antigen (PSMA)-targeting ligand, conjugated to the beta-emitting radioisotope lutetium Lu 177 (177Lu), with potential antineoplastic activity. Upon administration of lutetium Lu-177 PNT2002, the PNT2002 moiety targets and binds to PSMA-expressing tumor cells. Upon binding, PSMA-expressing tumor cells are destroyed by 177Lu through the specific delivery of beta particle radiation. PSMA, a tumor-associated antigen (TAA) and type II transmembrane protein, is expressed on the membrane of prostatic epithelial cells and overexpressed on the majority of prostate tumor cells. The FDA has also granted fast track designation to the prostate specific membrane antigen (PSMA)-targeted therapy, 177Lu-PNT2002, for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC). Currently, the drug is in Phase III stage of its development for the treatment of Metastatic Hormone Refractory Prostate Cancer.
* Opevesostat: Merck
Opevesostat is an oral, non-steroidal and selective inhibitor of the CYP11A1 enzyme discovered and developed by Orion for the treatment of hormone-dependent cancers, such as prostate cancer. By inhibiting CYP11A1 enzyme activity, ODM-208 is designed to suppress the production of all steroid hormones and their precursors that may activate the androgen receptor signaling pathway. . Currently, the drug is in Phase III stage of its development for the treatment of Metastatic Hormone Refractory Prostate Cancer.
* XL092: Exelixis
XL092 is a next-generation oral TKI that inhibits the activity of receptor tyrosine kinases implicated in cancer growth and spread, including VEGF receptors, MET, AXL and MER. These receptor tyrosine kinases are involved in both normal cellular function and in pathologic processes such as oncogenesis, metastasis, tumor angiogenesis, and resistance to multiple therapies, including immune checkpoint inhibitors. XL092 is currently being developed for the treatment of advanced solid tumors, including genitourinary cancers, as a monotherapy and in combination with immune checkpoint inhibitors. Currently, the drug is in Phase II stage of its development for the treatment of Metastatic Hormone Refractory Prostate Cancer.
* ZEN 3694: Zenith Epigenetics
ZEN-3694 is an orally bioavailable, potent, small molecule BET inhibitor that selectively binds to both bromodomains of the BET proteins. The drug candidate was discovered and developed from a BET bromodomain inhibitor platform. Bromodomain and Extra-Terminal domain (BET) family of proteins (BRD2, BRD3, BRD4, and BRDT) can bind acetylated lysines through their tandem bromodomains to promote gene transcription. BET bromodomain inhibitors (BETi) target super enhancers and inhibit several programs involved in tumorigenesis such as proliferation, metastasis, invasion, and immune evasion. Currently, the drug is in Phase II stage of its development for the treatment of Metastatic Hormone Refractory Prostate Cancer.
* CCS1477: CellCentric
Inobrodib is a small molecule inhibitor, taken orally as a capsule. It inhibits p300 and CBP through binding into the conserved bromodomain of the twin proteins. This impacts the expression of key cancer drivers, including MYC and IRF4. Different cancer indications can be targeted by inobrodib as monotherapy or in combination with existing standard of care drugs. Currently, the drug is in Phase I/II stage of its development for the treatment of Metastatic Hormone Refractory Prostate Cancer.
* KPT-8602: Karyopharm Therapeutics
Eltanexor (KPT-8602) is a second generation oral SINE compound. Eltanexor functions by binding to and inhibiting the nuclear export protein XPO1 (also called CRM1), leading to the accumulation of tumor suppressor proteins in the cell nucleus. Eltanexor has demonstrated minimal brain penetration in animals, which has been associated with reduced toxicities in preclinical studies while maintaining potent anti-tumor effects. Currently, the drug is in Phase I/II stage of its development for the treatment of Metastatic Hormone Refractory Prostate Cancer.
* JANX 007: Janux Therapeutics
JANX007 is lead novel Tumor Activated T Cell Engager (TRACTr). JANX007 is designed to target PSMA, a protein expressed in prostate cancer tumors and the vasculature of tumors and is in the clinic for the treatment of metastatic castration-resistant prostate cancer (mCRPC). The company designed PSMA-TRACTr drug candidate as a single-masked TRACTr in which the PSMA-binding domain is unmasked. The T cell-specific binding domain (CD3e) is masked to help minimize CRS. Currently, the drug is in Phase I stage of its development for the treatment of Metastatic Hormone Refractory Prostate Cancer.
* PF07248144: Pfizer
PF-07248144, developed by Pfizer, is a small molecule antineoplastic agent that functions as a histone acetyltransferase (HAT) inhibitor. By inhibiting HAT, it potentially disrupts gene expression and cellular processes that promote cancer growth. Its mechanism of action targets the modification of histones, a key process in epigenetic regulation. Currently, the drug is in Phase I stage of its development for the treatment of Metastatic Hormone Refractory Prostate Cancer.
Learn more about Metastatic Hormone Refractory Prostate Cancer Drugs opportunities in our groundbreaking Research and development projects @ Metastatic Hormone Refractory Prostate Cancer Unmet Needs [https://www.delveinsight.com/sample-request/metastatic-hormone-refractory-prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
The Metastatic Hormone Refractory Prostate Cancer Pipeline Report provides insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Metastatic Hormone Refractory Prostate Cancer with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Metastatic Hormone Refractory Prostate Cancer Treatment.
* Metastatic Hormone Refractory Prostate Cancer Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Metastatic Hormone Refractory Prostate Cancer Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Metastatic Hormone Refractory Prostate Cancer market.
Metastatic Hormone Refractory Prostate Cancer Companies
Lantheus, Merck, Exelixis, Zenith Epigenetics, CellCentric, Karyopharm Therapeutics, Janux Therapeutics, Pfizer, Tavanta Therapeutics, Telix Pharmaceuticals, Jiangsu HengRui Medicine, SOTIO, Antev Ltd., Syntrix Pharmaceuticals, Regeneron Pharmaceuticals, Madison Vaccines, Phosplatin Therapeutics, MacroGenics, RedHill Biopharma, Xencor and others.
Metastatic Hormone Refractory Prostate Cancer pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Oral
* Intravenous
* Subcutaneous
* Parenteral
* Topical
Metastatic Hormone Refractory Prostate Cancer Products have been categorized under various Molecule types such as
* Recombinant fusion proteins
* Small molecule
* Monoclonal antibody
* Peptide
* Polymer
* Gene Therapy
Stay informed about how we're transforming the future of Oncology @ Metastatic Hormone Refractory Prostate Cancer Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/metastatic-hormone-refractory-prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Metastatic Hormone Refractory Prostate Cancer Pipeline Report
* Coverage- Global
* Metastatic Hormone Refractory Prostate Cancer Companies- Lantheus, Merck, Exelixis, Zenith Epigenetics, CellCentric, Karyopharm Therapeutics, Janux Therapeutics, Pfizer, Tavanta Therapeutics, Telix Pharmaceuticals, Jiangsu HengRui Medicine, SOTIO, Antev Ltd., Syntrix Pharmaceuticals, Regeneron Pharmaceuticals, Madison Vaccines, Phosplatin Therapeutics, MacroGenics, RedHill Biopharma, Xencor and others.
* Metastatic Hormone Refractory Prostate Cancer Therapies- Enzalutamide, Patupilone, Prednisone, Docetaxel, Zactima (vandetanib), MDX-010, Denosumab, RAD001 , and others.
* Metastatic Hormone Refractory Prostate Cancer Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Metastatic Hormone Refractory Prostate Cancer Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Read the full details of Metastatic Hormone Refractory Prostate Cancer Pipeline on our website, @ Metastatic Hormone Refractory Prostate Cancer Emerging Drugs and Companies [https://www.delveinsight.com/sample-request/metastatic-hormone-refractory-prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Contents
* Introduction
* Executive Summary
* Metastatic Hormone Refractory Prostate Cancer: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Metastatic Hormone Refractory Prostate Cancer- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* PNT2002: Lantheus
* Mid Stage Products (Phase II)
* XL092: Exelixis
* Early Stage Products (Phase I)
* PF07248144: Pfizer
* Preclinical and Discovery Stage Products
* Inactive Products
* Metastatic Hormone Refractory Prostate Cancer Key Companies
* Metastatic Hormone Refractory Prostate Cancer Key Products
* Metastatic Hormone Refractory Prostate Cancer- Unmet Needs
* Metastatic Hormone Refractory Prostate Cancer- Market Drivers and Barriers
* Metastatic Hormone Refractory Prostate Cancer- Future Perspectives and Conclusion
* Metastatic Hormone Refractory Prostate Cancer Analyst Views
* Metastatic Hormone Refractory Prostate Cancer Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=metastatic-hormone-refractory-prostate-cancer-clinical-trial-pipeline-expands-as-180-companies-driving-innovation-in-the-therapeutics-delveinsight]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/metastatic-hormone-refractory-prostate-cancer-pipeline-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Metastatic Hormone Refractory Prostate Cancer Clinical Trial Pipeline Expands as 180+ Companies Driving Innovation in the Therapeutics | DelveInsight here
News-ID: 4390697 • Views: …
More Releases from ABNewswire

Motor Neuron Disease Treatment Pipeline Shows Strong Momentum as 180+ Pharma Com …
DelveInsight's, "Motor Neuron Disease Pipeline Insight 2025" report provides comprehensive insights about 180+ companies and 200+ pipeline drugs in Motor Neuron Disease pipeline landscape. It covers the Motor Neuron Disease pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Motor Neuron Disease pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Download DelveInsight's…

Non-Hodgkin's Lymphoma Clinical Trial Pipeline Shows Potential with Active Contr …
DelveInsight's, "Non-Hodgkin Lymphoma Pipeline Insights 2025" report provides comprehensive insights about 180+ companies and 200+ pipeline drugs in Non-Hodgkin Lymphoma pipeline landscape. It covers the Non-Hodgkin's Lymphoma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Non-Hodgkin's Lymphoma pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Dive into DelveInsight's comprehensive report today!…

Interstitial Lung Diseases Clinical Trial Pipeline Gains Momentum: 120+ Companie …
DelveInsight's "Interstitial Lung Diseases Pipeline Insight 2025" report provides comprehensive insights about 120+ companies and 120+ pipeline drugs in the Interstitial Lung Disease pipeline landscape. It covers the Interstitial Lung Diseases pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Interstitial Lung Diseases pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Discover…
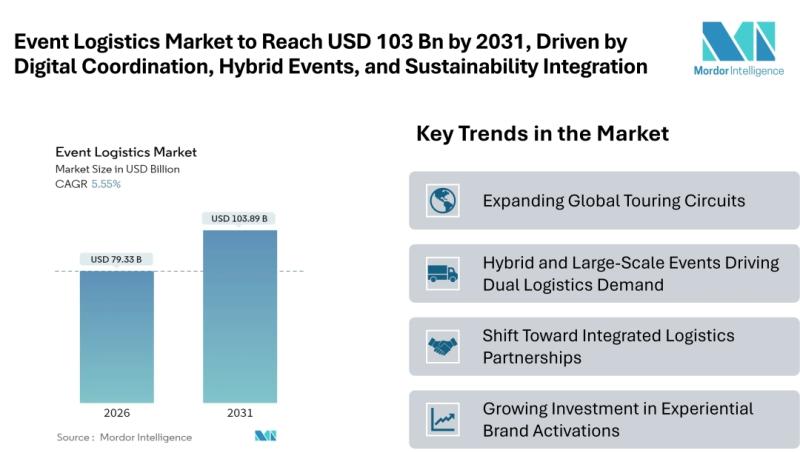
Event Logistics Market to Reach USD 103.89 Billion by 2031, Driven by Digital Co …
Mordor Intelligence has published a new report on the Event Logistics Market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Event Logistics Market Overview
According to Mordor Intelligence, the event logistics market size [https://www.mordorintelligence.com/industry-reports/global-event-logistics-market?utm_source=abnewswire] stood at USD 75.16 billion in 2025 and is projected to grow from USD 79.33 billion in 2026 to USD 103.89 billion by 2031, registering a CAGR of 5.55% during 2026-2031. Expansion is being driven…
More Releases for Metastatic
Key Trends Reshaping the Metastatic Bone Disease Market: Advancements in Targete …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Metastatic Bone Disease Market Size Growth Forecast: What to Expect by 2025?
The market size for metastatic bone disease has seen a quick expansion in the past few years. This market will increase from a value of $17.14 billion in 2024 to $18.88 billion in 2025, with a compound…
Emerging Trends Influencing The Growth Of The Metastatic Urothelial Carcinoma Ma …
We've updated all our reports with current data on tariff changes, trade developments, and supply chain shifts affecting key industries.
How Big Is the Metastatic Urothelial Carcinoma Market Size Expected to Be by 2034?
The market size for metastatic urothelial carcinoma has seen exponential growth recently. The market will expand from a worth of $1.18 billion in 2024 to $1.42 billion in 2025, with a compound annual growth rate (CAGR) of 20.2%.…
Key Influencer in the Metastatic Cancer Drugs Market 2025: Surging Prevalence Of …
How Are the key drivers contributing to the expansion of the metastatic cancer drugs market?
The escalation in the incidence of metastatic cancers will be a driving force for the expansion of the metastatic cancer drugs market. Metastatic cancer is a late-stage cancer that spreads across the body. As the number of metastatic cancer cases escalates, so does the creation of innovative drugs to treat various kinds of metastatic cancers. The…
Prominent Metastatic Breast Cancer Treatment Market Trend for 2025: Advancements …
Which drivers are expected to have the greatest impact on the over the metastatic breast cancer treatment market's growth?
The increasing occurrence of breast cancer is anticipated to boost the progression of the metastatic breast cancer treatment market. Breast cancer is a form of the disease that originates in the breast cells and is caused by abnormal cell growth in the breast, leading to the formation of a tumor. This alteration…
Major Force in the Metastatic Bone Disease Market 2025: Impact Of Bone Cancer Pr …
How Will the Metastatic Bone Disease Market Grow, and What Is the Projected Market Size?
The market size for metastatic bone disease has seen considerable growth in recent years. There is an expected increase from $17.14 billion in 2024 to $18.88 billion in 2025, with a compound annual growth rate (CAGR) of 10.1%. The substantial growth during the historical period can be credited to factors like rising aging population, heightened cancer…
Combination Therapy Strategies for Metastatic Cancer
Metastatic cancer, where cancer cells spread from the primary tumor to distant organs, presents significant treatment challenges. Combination therapy strategies have emerged as a powerful approach to enhance treatment efficacy, overcome resistance, and improve patient outcomes in metastatic cancer.
Download Report:
https://www.kuickresearch.com/report-cancer-antibody-combinations-therapy-cancer-antibody-combination-monoclonal-antibodies-combination-drug-conjugate-antibodies-combination
One of the primary goals of combination therapy in metastatic cancer is to target both the primary tumor and metastatic sites. This comprehensive approach can improve overall disease control…
