Press release
United States Epithelioid Sarcoma Treatment Market to Reach USD 2.66 Billion by 2031 at 9.1% CAGR, Driven by Targeted Therapies, Combination Treatments, and FDA-Approved EZH2 Inhibitors
The Epithelioid Sarcoma Treatment Market reached US$ 1.32 billion in 2023 and is expected to grow to around US$ 2.66 billion by 2031, expanding with a CAGR of approximately 9.1 % from 2024 to 2031 as advancements in targeted therapies and combination treatment strategies gain traction globally.Growth is supported by increasing demand across key treatment segments such as surgery, radiation therapy, chemotherapy, combination therapy, targeted drug therapy, and other therapeutic approaches, driven by rising clinical research, regulatory approvals for novel agents (including EZH2 inhibitors like tazemetostat), and growing need for effective options to manage this rare soft tissue sarcoma subtype. Epithelioid sarcoma characterized by the loss of the SMARCB1/INI1 gene and often affecting young adults presents significant treatment challenges, prompting heightened investment in therapeutic R&D, clinical trials, and multidisciplinary care pathways that improve patient outcomes. Expansion of oncology care infrastructure and enhanced awareness among healthcare providers further bolster broad adoption across hospitals and cancer treatment centers worldwide.
Download your exclusive sample report today (corporate email gets priority access):
https://www.datamintelligence.com/download-sample/epithelioid-sarcoma-treatment-market?sindhuri
Epithelioid Sarcoma Treatment Market: Competitive Intelligence
Novartis AG, Pfizer Inc., Johnson & Johnson (Janssen Pharmaceuticals), Eli Lilly and Company, Bristol‐Myers Squibb Company, Merck & Co., Inc., Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, AstraZeneca plc, and Pfizer Inc. Oncology, and others.
The Epithelioid Sarcoma Treatment Market is strongly driven by leading players such as Novartis, Pfizer, Janssen (Johnson & Johnson), Eli Lilly, and Bristol‐Myers Squibb, who develop and commercialize targeted therapies, chemotherapeutic agents, immunotherapies, and supportive cancer care solutions for managing epithelioid sarcoma a rare and aggressive soft tissue cancer. Their portfolios include small‐molecule inhibitors, monoclonal antibodies, checkpoint inhibitors, and combination regimens aimed at delaying progression, improving survival, and addressing metastatic disease. Rising incidence of soft tissue sarcomas, increased focus on rare cancer R&D, and growing clinical trial activity are key factors fueling market growth.
These companies' complementary strengths broad oncology pipelines and targeted agent leadership from Novartis and Pfizer; immuno‐oncology and checkpoint inhibitor portfolios from Bristol‐Myers Squibb and Merck; innovative combination therapy development from Janssen and Eli Lilly; and global clinical development and regulatory expertise from Takeda and AstraZeneca are enhancing competitive positioning. Strategic focus areas include late‐stage clinical trials and accelerated regulatory pathways, biomarker‐driven personalized therapy development, partnerships with academic research centers and rare disease consortia, expanded access programs, and investment in next‐generation modalities to improve outcomes for patients with epithelioid sarcoma globally.
Get Customization in the report as per your requirements:
https://www.datamintelligence.com/customize/epithelioid-sarcoma-treatment-market?sindhuri
Recent Key Developments - United States & North America
✅ September 2025: A U.S. pharmaceutical company launched a novel targeted therapy for epithelioid sarcoma designed to inhibit specific oncogenic pathways, aiming to improve progression‐free survival and expand options for patients with limited treatments.
✅ August 2025: The U.S. FDA approved a new immunotherapy for advanced epithelioid sarcoma, enhancing the treatment arsenal and allowing personalized therapy based on tumor profiling.
✅ July 2025: Clinical trials combining epigenetic modulators with chemotherapy agents were initiated at a U.S. cancer institute, exploring synergistic effects that could improve tumor control in refractory or metastatic epithelioid sarcoma.
Recent Key Developments - Japan & Asia‐Pacific
✅ August 2025: Japan's Ministry of Health, Labour and Welfare granted Orphan Drug Designation to tazemetostat (Tazverik®) for unresectable INI1‐negative epithelioid sarcoma after chemotherapy, signaling regulatory support for rare cancer drug development in Japan.
✅ Late 2025: A Phase II investigator‐initiated trial (TAZETTA) of tazemetostat for unresectable or metastatic epithelioid sarcoma continued enrollment in Japan, aiming to produce local efficacy data and support future approval expansion.
✅ 2025 Oncology Guidelines Update: Japanese oncology communities emphasized integration of targeted agents like EZH2 inhibitors into sarcoma management pathways at key conferences, reflecting evolving treatment strategies.
Recent Key Developments - Product & Clinical Pipeline
✅ Tazemetostat (TAZVERIK®) remains the first and only FDA‐approved targeted therapy for INI1‐negative epithelioid sarcoma, providing a benchmark for further development of molecular‐targeted options in this rare disease.
✅ Combination Therapy Trials: Multiple ongoing clinical investigations are exploring tazemetostat with immune checkpoint inhibitors and other systemic agents, aiming to improve outcomes compared with monotherapy in advanced disease.
✅ Pipeline Expansion: Broader soft tissue sarcoma research shows robust activity across >70 biotech players and 75+ candidate therapies, indicating increased interest in rare sarcoma treatment innovation, with some programs applicable to epithelioid sarcoma.
✅ 1. M&A / Strategic Developments
Regulatory & Strategic Support for Rare Oncology
Japan Orphan Drug Designation (Aug 2025) The Ministry of Health, Labour and Welfare (MHLW) in Japan granted orphan drug designation to tazemetostat (Tazverik®) for unresectable INI1‐negative epithelioid sarcoma, strengthening regulatory incentives (e.g., fee waivers, expedited review) and supporting commercial development in Asia‐Pacific.
Ongoing Investigator‐Led Trials A Phase II investigator‐initiated trial (TAZETTA) in Japan is enrolling patients with unresectable or metastatic epithelioid sarcoma to generate local efficacy data that may support expanded labeling or reimbursement discussions.
Industry Insight: While large pharmaceutical M&A specific to epithelioid sarcoma has not been reported recently, regulatory designations like orphan drug status and investigator‐initiated development reflect strategic positioning and collaboration with local oncology research communities.
✅ 2. New Product Launches & Development Activity
Existing Approved Therapy
Epizyme's Tazemetostat (Tazverik®) remains the only FDA‐approved targeted therapy for metastatic or locally advanced epithelioid sarcoma in patients aged ≥16 years who are not eligible for complete resection. This EZH2 inhibitor was granted accelerated approval based on response rates and duration of response, as a first‐in‐class targeted approach in this ultra‐rare sarcoma subtype.
Expanded International Recognition & Access
The Japan orphan designation works in tandem with global approvals, helping pave the way for regional labeling expansion and broader patient access in Asia.
Combination & Follow‐On Trials
Ongoing trials are evaluating tazemetostat in combination with other therapies (e.g., doxorubicin and immunotherapy agents) to improve outcomes beyond monotherapy in frontline and relapsed settings. These trials some adaptive and externally controlled are designed to overcome challenges in rare cancer research where large randomized studies are infeasible.
✅ 3. R&D & Innovation Trends
Precision Oncology & Molecular Stratification
Epithelioid sarcoma's biology characterized by INI1 loss and EZH2 pathway alterations has driven the advent of targeted therapies like EZH2 inhibitors that specifically exploit this vulnerability. Molecular diagnostics (e.g., next‐generation sequencing) are increasingly used to confirm eligibility for targeted agents and precision clinical trials.
Next‐Generation Trial Designs
Adaptive and basket trial designs are gaining traction. These approaches combine histology‐agnostic cohorts and external controls to accelerate development in ultra‐rare diseases, reduce patient burden, and create efficient data pathways for regulatory decision‐making.
Digital & AI‐Enabled Diagnostics
Digital pathology and AI‐assisted morphological analysis tools are being incorporated into clinical workflows and trial recruitment to speed diagnosis, identify rare histological features, and match patients to appropriate trials particularly valuable in conditions where every patient counts.
Segments Covered in the Epithelioid Sarcoma Treatment Market :
By Type
The market is segmented into Distal Type 60% and Proximal Type 40%, with distal type dominating due to its higher prevalence, particularly in young adults and extremity-associated cases. Proximal type, although less common, is associated with more aggressive progression and higher recurrence rates, driving demand for specialized treatment approaches. Increasing awareness and early detection initiatives support growth across both types.
By Treatment
Treatments include Surgery 35%, Combination Therapy 20%, Targeted Drug Therapy 15%, Radiation Therapy 10%, Chemotherapy 10%, and Others 10%, with surgery dominating as the primary approach for tumor removal and disease management. Combination therapies are growing with multi-modal approaches to reduce recurrence and improve survival outcomes. Targeted drug therapies are emerging rapidly due to advances in precision oncology. Radiation and chemotherapy maintain supportive roles, particularly in aggressive or recurrent cases. Continuous R&D in novel therapies supports market expansion.
By End-User
End-users include Hospitals 50%, Oncology Centers 30%, Ambulatory Surgical Centers 15%, and Others 5%, with hospitals dominating due to advanced surgical facilities, access to multi-disciplinary oncology teams, and high patient volume. Oncology centers focus on targeted therapies and clinical trial participation. Ambulatory surgical centers are expanding for less invasive procedures. Rising awareness and centralized cancer care initiatives drive end-user adoption.
Buy Now & Unlock 360° Market Intelligence:
https://www.datamintelligence.com/buy-now-page?report=epithelioid-sarcoma-treatment-market?sindhuri
By Region
North America - 40% Share
North America leads with 40% share, driven by advanced oncology infrastructure, early detection programs, and high adoption of targeted therapies. Surgery and combination therapies dominate. Hospitals and oncology centers are primary end-users. Ongoing clinical trials and research funding support growth.
Europe - 25% Share
Europe holds 25% share, supported by strong healthcare systems, multi-disciplinary cancer centers, and increasing awareness of rare sarcomas. Surgery remains the mainstay, while targeted drug therapies are expanding. Hospitals and oncology centers are key end-users.
Asia Pacific - 20% Share
Asia Pacific accounts for 20% share, driven by rising cancer awareness, improved healthcare infrastructure, and increasing access to specialized oncology treatments in countries such as Japan, China, and India. Distal type cases dominate. Hospitals lead end-user adoption.
Request for 2 Days FREE Trial Access:
https://www.datamintelligence.com/reports-subscription?sindhuri
✅ Competitive Landscape
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Consumer Behavior & Demand Analysis
✅ Import-Export Data Monitoring
✅ Live Market & Pricing Trends
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release United States Epithelioid Sarcoma Treatment Market to Reach USD 2.66 Billion by 2031 at 9.1% CAGR, Driven by Targeted Therapies, Combination Treatments, and FDA-Approved EZH2 Inhibitors here
News-ID: 4390217 • Views: …
More Releases from DataM Intelligence 4Market Research LLP

Biomass Briquette Market to Grow at 7% CAGR from 2025 to 2031, Led by North Amer …
The Biomass Briquette Market is expected to expand at a CAGR of about 7% from 2025 to 2031 as industries and households increasingly adopt sustainable and eco‐friendly solid fuel alternatives to traditional fossil fuels, driven by rising energy demand and environmental concerns.
Growth is supported by increasing demand across key applications such as industrial fuel, power generation, cooking fuel, and residential heating, driven by abundant agricultural and forestry residues that are…

Protein Engineering Market to Grow at 15.6% CAGR from 2025 to 2033, Driven by No …
The global protein engineering market reached US$ 2,854.54 Million in 2024 and is expected to reach US$ 8,646.10 Million by 2033, growing at a CAGR of 15.6 % from 2025 ro 2033 as demand for tailored biological solutions accelerates across pharmaceuticals, biotechnology, diagnostics, and industrial applications.
Growth is supported by increasing adoption of advanced protein modification, design and synthesis techniques to develop high‐efficacy therapeutics, enzymes, and biomaterials, driven by rising…

Substation Automation Market to Reach USD 67B by 2031 at 6.5% CAGR | Driven by R …
According to DataM Intelligence, the Substation Automation Market reached USD 41 billion in 2022 and is expected to reach USD 67 billion by 2031, growing at a CAGR of 6.5% during the forecast period (2024-2031).The global Substation Automation Market is undergoing a significant transformation as utilities and grid operators modernize aging infrastructure and transition toward digital substations. Substation automation systems (SAS) integrate intelligent electronic devices (IEDs), communication networks, SCADA platforms,…
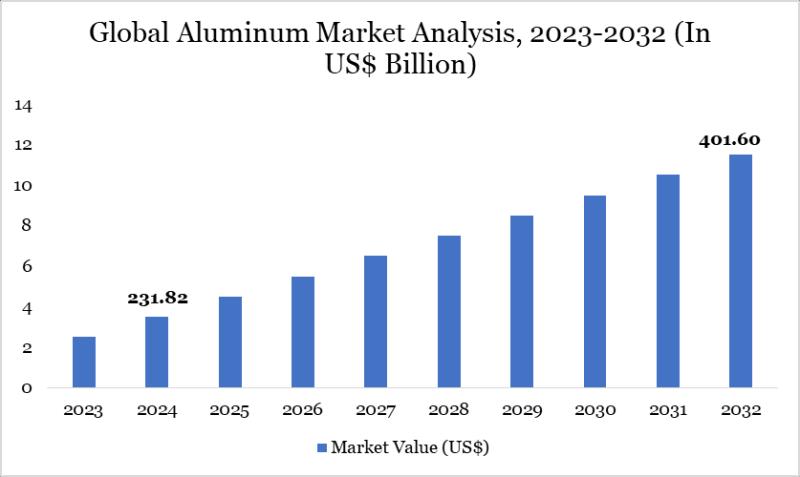
Aluminum Market to Reach US$ 401.60 Billion by 2032 Driven by Infrastructure Gro …
The Aluminum Market reached US$ 231.82 billion in 2024 and is expected to reach US$ 401.60 billion by 2032, growing at a CAGR of 7.11% during the forecast period 2025-2032.
Growth is driven by rising demand across construction, automotive, aerospace, packaging, and electrical industries, where aluminum is valued for its lightweight properties, corrosion resistance, high strength-to-weight ratio, and recyclability. Increasing focus on vehicle lightweighting, electric vehicle production, and sustainable packaging solutions…
More Releases for Epithelioid
United States Epithelioid Sarcoma Treatment Market 2031 | Growth Drivers, Key Pl …
Market Size and Growth
The global epithelioid sarcoma treatment market reached US$ 1.32 billion in 2023 and is expected to reach US$ 2.66 billion by 2031 growing with a CAGR of 9.1% during the forecast period 2024-2031.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/epithelioid-sarcoma-treatment-market?sb
Key Development:
United States: Recent Epithelioid Sarcoma Treatment Developments
✅ In January 2026, the FDA granted accelerated approval of tazemetostat (Tazverik®) as…
Perivascular Epithelioid Cell Tumor (PEComa) Market Forecast 2025-2034
Market Overview
The global Perivascular Epithelioid Cell Tumor (PEComa) Market was valued at approximately USD 0.28 billion in 2024 and is projected to reach USD 0.52 billion by 2034, growing at a CAGR of around 6.5%. Market growth is driven by increasing awareness of rare tumors, advancements in targeted therapies, and improving diagnostic capabilities.
Download Full PDF Sample Copy of Market Report
https://exactitudeconsultancy.com/request-sample/70959
Key Market Drivers
• Rising Incidence of Rare Tumors: Increased detection of…
Epithelioid Sarcoma Market is expected to reach USD 1.2 billion by 2034
Epithelioid sarcoma is a rare and aggressive form of soft tissue sarcoma that typically affects young adults and adolescents. Despite being classified as a soft tissue cancer, it is notorious for its high recurrence rate and metastasis, often posing challenges in treatment and long-term survival. Standard therapies such as surgical resection and chemotherapy have been the mainstay, but recent advances in targeted therapies, immunotherapies, and rare disease research incentives are…
Perivascular Epithelioid Cell Tumor Market Size, Share and Growth Report, 2034
Introduction
Perivascular epithelioid cell tumors (PEComas) are a rare group of tumors characterized by the presence of epithelioid cells and smooth muscle components. These tumors can arise in various organs, including the lungs, kidneys, liver, and soft tissues, and are often classified as benign or malignant based on their characteristics. While PEComas are uncommon, they present unique challenges for diagnosis and treatment due to their heterogeneous nature. The tumors can exhibit…
Epithelioid Sarcoma Treatment: Advancements and Emerging Trends in Rare Cancer C …
Epithelioid sarcoma is a rare type of soft tissue cancer that primarily affects young adults and tends to appear in the extremities, such as hands and arms. This cancer type is known for its challenging diagnosis, high recurrence rate, and complex treatment requirements. Because epithelioid sarcoma is a rare and aggressive form of cancer, its treatment landscape has evolved rapidly in recent years, bringing new therapies and approaches to patients.…
Epithelioid Sarcoma Treatment Market: A Comprehensive Overview
1. Introduction
Epithelioid sarcoma (ES) is a rare and aggressive soft tissue sarcoma, predominantly affecting young adults. It has a propensity for recurrence and metastasis, often posing significant challenges for effective treatment. The complexities surrounding epithelioid sarcoma have driven innovations in the treatment landscape, sparking considerable growth and evolution in the global market for ES therapies. The global epithelioid sarcoma treatment market is shaped by several factors including advancements in therapeutic…