Press release
ePRO, E-Patient Diaries and eCOA Market Forecast 2026-2033: 15.3% CAGR Growth Outlook by Persistence Market Research
EPRO, E-patient Diaries and eCOA Market: Trends, Insights, and ForecastsThe global ePRO (electronic Patient-Reported Outcomes), e-patient diaries, and eCOA (electronic Clinical Outcome Assessments) market is witnessing significant growth, fueled by increasing adoption of digital health technologies, decentralized clinical trials, and the growing need for real-time, reliable patient data collection. As of 2026, the global market is expected to reach USD 2.9 billion, and it is forecasted to grow to USD 7.9 billion by 2033, with a compound annual growth rate (CAGR) of 15.3%. This growth is driven by a combination of factors including technological advancements, regulatory acceptance, and the need for more efficient clinical trial methodologies. In this article, we will explore the key drivers, trends, market segmentation, and future opportunities within the ePRO, e-patient diaries, and eCOA market.
Download Your Free Sample & Explore Key Insights: https://www.persistencemarketresearch.com/samples/11506
Overview of the Market
The ePRO, e-patient diaries, and eCOA market is primarily driven by the need for accurate, real-time data capture during clinical trials. Traditional paper-based methods for patient-reported outcomes (PRO) and clinical assessments have become increasingly outdated due to inefficiencies such as transcription errors and recall bias. Electronic solutions offer more accurate and timely data, improving trial reliability and compliance. These tools are especially critical in decentralized and hybrid clinical trials, where data can be collected remotely, reducing the need for in-person visits.
Key growth drivers in this market include the increasing complexity of clinical trials, the rapid adoption of decentralized clinical trial models, regulatory support for patient-centric data, and the growing use of mobile and cloud technologies. North America remains the largest regional market for ePRO, e-patient diaries, and eCOA, owing to its well-established research infrastructure and robust regulatory support. However, Asia-Pacific is the fastest-growing region, with emerging markets such as China, India, and South Korea rapidly adopting digital health solutions to support growing clinical research activity.
Key Highlights from the Report
• The global ePRO, e-patient diaries, and eCOA market is projected to grow from USD 2.9 billion in 2026 to USD 7.9 billion by 2033.
• The market is expected to register a CAGR of 15.3% from 2026 to 2033.
• ePRO solutions held a 42.9% market share in 2025, making them the dominant segment.
• North America is the leading geographical region, accounting for 43.3% of the market share in 2025.
• Asia-Pacific is the fastest-growing region, driven by increasing trial volumes and healthcare digitization.
• Wearables and mobile integration present significant growth opportunities for the market.
Market Segmentation
The ePRO, e-patient diaries, and eCOA market can be segmented in a variety of ways, including by product type, end-user, and technology.
By Product Type
ePRO solutions are the dominant segment, accounting for a significant share of the market. These solutions are widely used for capturing patient-reported outcomes, which are essential for evaluating the effectiveness of medical interventions in clinical trials. The use of ePRO reduces errors associated with paper diaries, such as transcription mistakes and recall bias, by capturing data in real-time through electronic devices. This segment is expected to grow as regulatory bodies such as the U.S. FDA increasingly recognize ePRO as a legitimate source of clinical trial data.
eCOA is another significant segment, focusing on electronic tools used for assessing clinical outcomes. These solutions provide a standardized approach to assessing clinical endpoints such as disease progression, symptom severity, and quality of life. eCOA tools are highly effective in both clinical trials and post-market studies, as they enable real-time data collection and facilitate monitoring from remote locations. The adoption of eCOA is expected to increase, particularly with the rise of hybrid and decentralized clinical trials.
By End-User
The end-user segment includes pharmaceutical companies, contract research organizations (CROs), academic research institutions, and healthcare providers. Pharmaceutical companies are the largest end-users of ePRO and eCOA solutions due to the growing complexity of clinical trials and the need for accurate, real-time data capture. CROs are also significant players, as they often manage clinical trials on behalf of pharmaceutical companies, and they are increasingly adopting digital solutions to improve trial efficiency.
Academic institutions, particularly those conducting clinical research, also represent a key segment. These institutions are increasingly leveraging ePRO and eCOA solutions to streamline data collection and enhance patient engagement in clinical trials. Healthcare providers are gradually adopting these solutions as well, particularly in the context of real-world evidence studies and patient monitoring programs.
By Modality
Mobile devices, such as smartphones and tablets, dominate the modality segment due to their widespread adoption and user-friendliness. Patients are more likely to engage with mobile platforms, as they are convenient and accessible, allowing patients to report outcomes remotely at any time. This is especially critical in decentralized trials, where patients may be located in remote areas or across multiple geographical locations.
Cloud-based platforms are integral to the deployment of mobile ePRO and eCOA solutions. They allow for the seamless integration of data, enabling real-time monitoring, regulatory compliance, and quick data transfer. These platforms also offer scalability and flexibility, making them an attractive option for global clinical trials.
Get Custom Insights Designed for Your Business: https://www.persistencemarketresearch.com/request-customization/11506
Regional Insights
North America
North America holds the largest share of the ePRO, e-patient diaries, and eCOA market, accounting for 43.3% of the market share in 2025. This dominance is attributed to several factors, including a highly developed healthcare infrastructure, the presence of major pharmaceutical companies, and regulatory support from agencies like the FDA. North America has long been a leader in clinical trials, with many of the world's top clinical research organizations (CROs) based in the region. Moreover, the FDA's clear guidelines for the use of ePRO and eCOA in clinical trials have encouraged widespread adoption.
The U.S. particularly stands out as a leader in adopting decentralized and hybrid trial models, with over 74% of Phase III trials utilizing eCOA/ePRO systems. As clinical trials become more complex and patient-centric, the adoption of ePRO and eCOA solutions is expected to continue to rise in North America.
Europe
Europe is another key market for ePRO, e-patient diaries, and eCOA solutions. The region's mature clinical research ecosystem, along with its strong regulatory framework, supports the widespread adoption of digital health solutions. The European Medicines Agency (EMA) encourages patient-centric approaches to clinical trials, further boosting the demand for electronic outcome assessments. In addition, GDPR regulations ensure that patient data is securely captured, fostering trust in digital solutions.
Multinational clinical trials in Europe often involve diverse patient populations across multiple languages. eCOA platforms are particularly beneficial in these trials, as they enable standardized data collection across various regions and languages. As of 2025, approximately 68% of large-scale clinical trials in Europe use eCOA solutions, highlighting the growing importance of these platforms in the region.
Asia-Pacific
Asia-Pacific is the fastest-growing market for ePRO, e-patient diaries, and eCOA solutions. The region has seen significant growth in clinical trial activity, particularly in countries like China, India, Japan, and South Korea. The increasing adoption of mobile and cloud technologies, along with government investments in digital health infrastructure, has facilitated the rapid expansion of digital health tools.
The region's growing focus on healthcare digitization is evident, with more than 59% of new clinical trials in Asia-Pacific incorporating digital data capture, including ePRO and eCOA solutions. High smartphone penetration in countries like India and China has also contributed to the success of bring-your-own-device (BYOD) models, enabling widespread participation in decentralized trials.
Market Drivers
The primary driver for the ePRO, e-patient diaries, and eCOA market is the increasing complexity of clinical trials. As trials become more complex, with larger patient populations and more diverse study endpoints, the need for accurate, real-time data collection becomes more critical. Digital health solutions like ePRO and eCOA enable researchers to capture patient-reported outcomes and clinical data efficiently and accurately.
The shift toward decentralized clinical trials (DCTs) is another major market driver. DCTs allow for greater patient participation and more flexible trial designs, eliminating the need for in-person visits. This has been particularly important during the COVID-19 pandemic and is expected to remain a dominant trend moving forward. Digital tools like ePRO and eCOA play a central role in DCTs by enabling remote data collection and patient engagement.
Regulatory support for patient-centric data is also driving the market. Agencies like the FDA and EMA are increasingly recognizing the importance of patient-reported outcomes in clinical trials, which has led to the formal approval of ePRO and eCOA systems as valid sources of clinical data. This regulatory acceptance has encouraged sponsors to adopt these technologies more widely.
Market Restraints
Despite the growth opportunities, the ePRO, e-patient diaries, and eCOA market faces some significant challenges. The most notable restraint is the high initial cost of implementing these technologies. Clinical trial software deployments often require substantial investment in software licensing, customization, validation, and staff training. Smaller biotechnology firms and academic research institutions may find these upfront costs prohibitive, leading to delays in adopting these solutions.
Moreover, the ongoing maintenance and validation of these systems to ensure compliance with regulatory standards (such as 21 CFR Part 11 and HIPAA) can add to the financial burden. These barriers are particularly challenging for cost-sensitive organizations, limiting the widespread adoption of ePRO and eCOA solutions.
Market Opportunities
The integration of wearable devices and remote patient monitoring (RPM) tools presents a significant opportunity for the ePRO, e-patient diaries, and eCOA market. Wearables provide continuous, objective data such as activity levels, vitals, and biomarkers, which can be integrated with ePRO/eCOA systems to enhance the accuracy and completeness of clinical trial data. This integration can improve patient engagement and reduce reliance on recall-based reporting.
Another key opportunity lies in the expansion of digital health solutions in emerging markets. Countries in the Asia-Pacific and Latin America regions are increasingly adopting digital health technologies, driven by factors such as mobile phone penetration, government investments in healthcare infrastructure, and the growing outsourcing of clinical trials to these regions. These markets present significant growth potential for ePRO, e-patient diaries, and eCOA solutions.
Checkout Now & Download Complete Market Report: https://www.persistencemarketresearch.com/checkout/11506
Company Insights
Several key players dominate the ePRO, e-patient diaries, and eCOA market. These companies are at the forefront of innovation, offering solutions that support digital data capture, real-time monitoring, and regulatory compliance in clinical trials.
• Medidata Solutions (Dassault Systèmes)
• Signant Health
• IQVIA Holdings, Inc.
• Clario (formerly ERT)
• Oracle Corporation
• Parexel International Corporation
• ICON plc
• Kayentis
• CRF Health
• OmniComm Systems
• Medable Inc.
Recent Developments
In December 2025, the npj Digital Medicine journal published a study on best practices for event-driven electronic diaries (EDeDs) in clinical trials, emphasizing the importance of usability, compliance monitoring, and clear responsibilities for data reporting.
Medidata Solutions, in partnership with several leading pharmaceutical companies, announced the successful integration of AI-powered analytics into its ePRO/eCOA platforms to improve patient data analysis and decision-making.
Conclusion
The ePRO, e-patient diaries, and eCOA market is poised for substantial growth, driven by technological innovations, regulatory support, and the shift towards decentralized and hybrid clinical trial models. As the market continues to expand, the integration of mobile devices, wearables, and cloud-based solutions will play a pivotal role in enhancing trial data quality, improving patient engagement, and streamlining the clinical trial process. While challenges such as high initial costs remain, the opportunities for innovation and growth, particularly in emerging markets and through the adoption of wearables, make this an exciting sector to watch in the coming years.
Contact Us:
Persistence Market Research
Second Floor, 150 Fleet Street, London, EC4A 2DQ, United Kingdom
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release ePRO, E-Patient Diaries and eCOA Market Forecast 2026-2033: 15.3% CAGR Growth Outlook by Persistence Market Research here
News-ID: 4387323 • Views: …
More Releases from Persistence Market Research

Gene Expression Analysis Market Growth Projected at 8.4% CAGR, Valuation to Reac …
The gene expression analysis market is rapidly evolving, driven by advancements in genomics technologies and the increasing demand for precision medicine. Over the last decade, the field has transformed from a niche research segment to a core component in diagnostics, drug development, and clinical applications. This transformation has been fueled by innovations in sequencing technologies, the rising prevalence of chronic diseases, and the expanding role of gene expression profiling in…

Biosimilar Insulin Market to Reach US$ 6.0 Bn by 2033, Expanding at 14.9% CAGR | …
The biosimilar insulin market has witnessed significant growth in recent years, driven by increasing global diabetes prevalence, rising healthcare costs, and the demand for affordable alternatives to branded insulin therapies. As more people around the world develop type 1 and type 2 diabetes, the need for effective and cost-efficient insulin options has become more critical. This article provides an in-depth analysis of the biosimilar insulin market, including key growth drivers,…
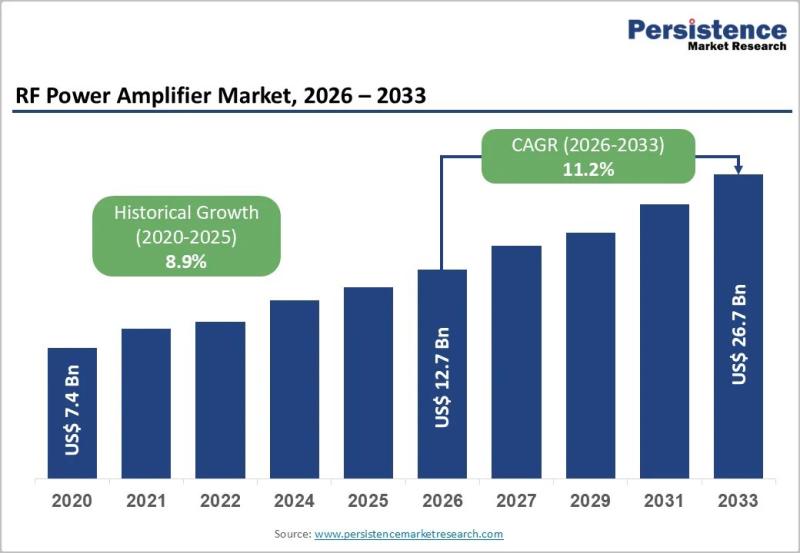
RF Power Amplifier Market Accelerates on 5G, GaN, and Defense Modernisation
RF Power Amplifier Market Overview and Growth Outlook
The global RF Power Amplifier Market is poised for sustained double-digit growth, projected to rise from US$ 12.7 billion in 2026 to US$ 26.7 billion by 2033, reflecting a strong CAGR of 11.2% during the forecast period. This expansion is underpinned by structural demand across telecommunications infrastructure, aerospace and defense modernization, and next-generation satellite communication (SATCOM) systems. As mobile data traffic surges and…
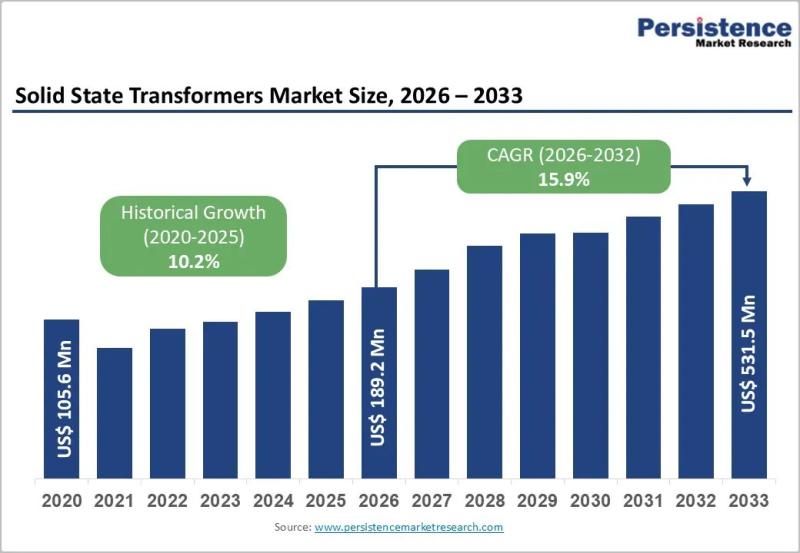
Smart Grid Expansion Driving Solid State Transformers Demand
Solid State Transformers Market Overview and Growth Outlook
The global Solid State Transformers Market is entering a high-growth phase, driven by rapid grid modernization and the accelerating shift toward renewable energy. Valued at US$ 189.2 million in 2026, the market is projected to reach US$ 531.5 million by 2033, expanding at a robust CAGR of 15.9% during the forecast period. The rising need for intelligent power distribution, bidirectional energy flow, and…
More Releases for PRO
WineCork Pro Reviews: Truth About WineCork Pro Revealed!!
There's nothing quite like the anticipation of opening a fine bottle of wine; whether you're celebrating a special moment, hosting friends, or winding down after a long day. But too often, that moment of joy is ruined by a stubborn cork, a broken opener, or the all-too-familiar embarrassment of struggling in front of guests. That's where WineCork Pro comes in; the smart, sleek solution that transforms wine opening from a…
BuzzZapper Pro Reviews: Is Buzz Zapper Pro Worth It?
BuzzZapper Pro Reviews: Is Buzz Zapper Pro Worth It?
If you've ever tried enjoying a quiet evening outdoors only to get eaten alive by mosquitoes, you'll understand exactly where I'm coming from. I've tried everything, from citronella candles to bug sprays that smell like a science experiment gone wrong. Some worked a little, most didn't, and all of them felt like a temporary fix.
That's when I came across the BuzzZapper Pro,…
Balmorex Pro: Does Balmorex Pro Really Works? Read The Balmorex Pro Reviews
Introduction
Joint and muscle aches can result from everyday wear and tear, aging, and poor nutrition. Whatever the reason, pain and ache issues are less than desirable. Who wants to suffer, rely on canes, or undergo surgery? No one, if they have a say in it.
With no guarantee of surgeries being successful and a high-risk rate, people have begun to rely on natural remedies that can prevent damage and start the…
ScrubClean Pro Reviews: Truth About ScrubClean Pro Scrubber Revealed
Based on ScrubClean Pro Reviews, it is one of the best spin power scrubber that is fairly priced. It is highly efficient with 4.9 stat ratings from the real users.
In this review, we are going to detail everything you might want to know about this innovative cleaning tools that is catching the market by storms. It is new but the amazing thing is that it is the number consumer choice.…
Pro Power Saver Reviews: is Pro Power Saver Legit? Read Pro Power Saver Consumer …
In recent years, the rising cost of energy bills has become a significant concern for both renters and homeowners in the United States. As the cost of living continues to increase, many individuals and families are searching for effective ways to reduce their monthly expenses. In response to this demand, a new device called Pro Power Saver has emerged as a trending solution in the country.
Pro Power Saver is an…
Pro Power Save (USA Update) Is Pro Power Save Legit? Pro Power save Reviews Cons …
Pro Power Save also known as Miracle Watt Energy Saver, the most trending energy saver in the United States with a stellar customer review rating of 4.8 out of 5.0. If you're tired of dealing with high electricity bills and want to save money, then this device might be the solution you've been searching for.
Pro Power Save is designed to help millions of users reduce their energy consumption when…
