Press release
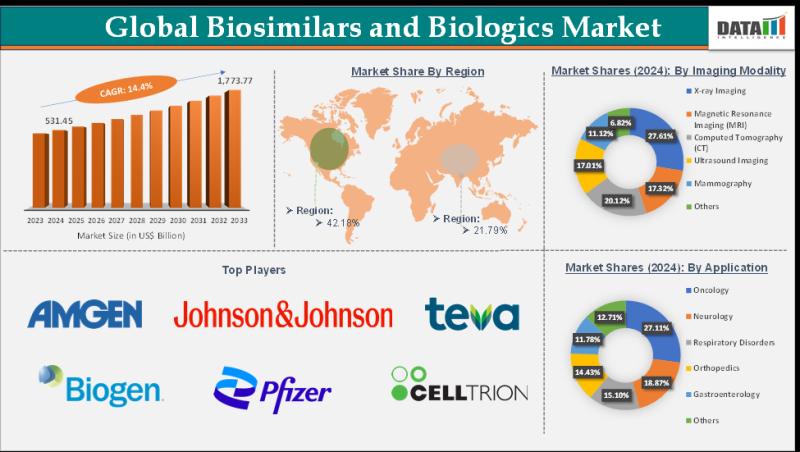
Biosimilars and Biologics Market is projected to reach US$ 1.77 trillion by 2033, growing at a CAGR of 14.4%, North America is expected to dominate the market with a 42.8% share
Biosimilars and Biologics Market size reached US$ 531.45 Billion in 2024 and is expected to reach US$ 1,773.77 Billion by 2033, growing at a CAGR of 14.4% during the forecast period 2025-2033.The biosimilars and biologics market is growing because the rise in chronic diseases and aging populations increases demand for advanced therapies, while patent expiries allow more affordable biosimilars to enter, expanding patient access and reducing healthcare costs.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/biosimilars-and-biologics-market?prtk
United States: Key Industry Developments (2026-2025)
✅ January 2026: FDA released a draft guidance to streamline biosimilar development, proposing to ease clinical study requirements and modernize interchangeability standards to expand competition and reduce costs.
✅ October 2025: U.S. regulators announced plans to expedite approvals for biosimilars, signaling reduced human clinical testing and regulatory simplification to lower development barriers and boost market entry.
✅ January 2026: Filkri (filgrastim-laha) received FDA approval as a biosimilar to Neupogen, expanding lower-cost options for neutropenia treatment.
✅ December 2025: Multiple biosimilars including Boncresa and Oziltus (denosumab-mobz) and Nufymco (ranibizumab-leyk) were approved, broadening patient access for bone health and ophthalmologic conditions.
✅ November 2025: Armlupeg (pegfilgrastim-unne) and Poherdy (pertuzumab-dpzb) launched, adding long-acting supportive care and oncology biosimilar options to the U.S. market.
Japan: Key Industry Developments (2025)
✅ September 2025: Japan's PMDA approved biosimilars for golimumab and tocilizumab, enhancing treatment options for autoimmune and inflammatory conditions through locally regulated biologic alternatives.
✅ January 2025: Biocon Biologics' ustekinumab biosimilar (Stelara® BS) received PMDA approval, marking expanded biosimilar availability in Japan for psoriasis and related conditions.
Biosimilars and Biologics Market Recent M&A activities:-
→ On December 6, 2025, Biocon Limited announced the full integration of Biocon Biologics Limited (BBL) as a wholly owned subsidiary, acquiring the remaining minority stakes from Serum Institute Life Sciences, Tata Capital Growth Fund II and Activ Pine LLP via share swaps and acquiring Viatris' (Mylan) residual stake for cash/share consideration. The transaction values the biosimilars business at approximately $5.5 billion, with Viatris stake consideration of about $815 million (including $400 million cash and $415 million in share swap)**. The integration is expected to be completed by March 31, 2026.
→ On December 8, 2025, Sandoz completed its acquisition of Just-Evotec Biologics EU SAS from Evotec SE, including the Toulouse biologics development and manufacturing site. The upfront cash consideration was approximately $350 million, with potential additional license fees and development revenues expected to bring total potential consideration to more than $650 million plus royalties under the agreement.
→ On November 4, 2025, Sandoz and Evotec SE signed the definitive agreement (prior to closing) for Sandoz to acquire 100 % of Just-Evotec Biologics EU SAS, including an indefinite technology license to Evotec's continuous manufacturing platform, with an upfront transaction value of $350 million plus future fees/milestones valued at less then $300 million.
Biosimilars and Biologics Market key Players:-
Amgen, Sanofi, Pfizer, Eli Lilly, Novartis, Viatris, Biocon Biologics, Roche, Celltrion and Teva.
Top 5 Key Players Analysis:-
Pfizer - Commands 20%+ of the global biologics and biosimilars market, leveraging a strong biosimilar portfolio and global distribution footprint. Its core strength is expansive R&D and strategic acquisitions (e.g., Hospira) that bolster monoclonal antibody and protein-based biosimilar offerings.
Roche - Holds roughly 29% of the global biologics market, making it one of the industry's largest originator biologics players. Its core strength lies in innovative high-value biologics, especially monoclonal antibodies across oncology and immunology.
Amgen - Accounts for about 14% of market share in the combined biologics and biosimilars landscape, supported by a broad pipeline and key biosimilar approvals. It excels in recombinant proteins and oncology/immunology biologics backed by deep manufacturing expertise.
Novartis (Sandoz) - Represents around 10% of global market share via its biosimilars division, with strong adoption in Europe and expanding reach globally. Its core strength is diversified biosimilar assets across multiple therapeutic areas and extensive commercialization networks.
Eli Lilly - Holds approximately 12% of the biologics and biosimilars market, driven by leadership in insulin and monoclonal antibody biosimilars. Its strength stems from therapeutic breadth in diabetes, oncology and immunology biologics and growing biosimilar portfolio.
Biosimilars and Biologics Market Top Technological Partnerships (2026 & 2025):-
Biocon Biologics & Civica, Inc. Expanded Strategic Collaboration on Insulin Biosimilars (Oct 2025):
Biocon Biologics expanded its multi-year partnership with Civica to manufacture and supply Insulin Glargine (a biosimilar) in the United States, where Civica will commercialize it through an exclusive private-label arrangementboosting affordable insulin access while leveraging Biocon's manufacturing capabilities.
Biocon Biologics & Civica Earlier Collaboration on Insulin Aspart (Mar 2025):
In a prior phase of their collaboration, Biocon Biologics supplied Insulin Aspart drug substance to Civica for manufacturing and commercialization in the U.S., marking an important step in expanding biosimilar insulin availability.
Alvotech Supply & Commercialization Deals for Canada, Australia & New
Zealand (Early 2026):
Alvotech entered into supply and commercialization agreements covering multiple biosimilar candidates across Canada and Australia/New Zealand, strengthening its global biosimilar footprint in these regions.
Formycon & MS Pharma Exclusive Commercialization Partnership for Keytruda® Biosimilar in MENA (Dec 2025):
Formycon signed an agreement with MS Pharma for exclusive commercialization of a Keytruda® biosimilar candidate (FYB206) tailored to the Middle East & North Africa (MENA) region, advancing regional access to oncology biosimilars.
Bio-Thera Solutions & SteinCares Expanded Latin America Biosimilar Commercialization (2025):
Bio-Thera Solutions and SteinCares strengthened their partnership to commercialize an additional proposed biosimilar (e.g., dupilumab) across Latin America, combining development and distribution expertise to broaden patient access.
This report addresses:
Market intelligence to enable effective decision making
Market estimates and forecasts from 2024-2032
Growth opportunities and trend analyses
Segment and regional revenue forecasts for market assessment
Competition strategy and market share analysis
Product innovation listing for you to stay ahead of the curve
COVID-19's impact and how to sustain in these fast-evolving markets
Buy Now & Unlock 360° Market Intelligence:https://www.datamintelligence.com/buy-now-page?report=biosimilars-and-biologics-market?prtk
Biosimilars and Biologics Market Drivers :-
Expansion Figure: The biosimilars market is projected to reach approximately $50 billion by the end of 2026, driven by a CAGR of nearly 15%.
Market Indicator: In the U.S. alone, biosimilar savings are expected to exceed $30 billion annually by 2026 as competition drives down the price of high-cost specialty drugs.
Adoption Rate: Interchangeable biosimilars (such as those for Humira and Stelara) have seen a 40% faster uptake in 2025 compared to non-interchangeable versions launched in previous years.
Efficiency Gain: Automated substitution at the pharmacy level has reduced the administrative burden on healthcare providers, leading to a 25% increase in biosimilar prescriptions within the first six months of 2025.
Segment Growth: Oncology-related biosimilars now account for over 35% of the total market share, with multiple biosimilar versions of Keytruda and Opdivo currently in late-stage clinical trials or pending 2026 launches.
Patient Access: Increased competition in the TNF-inhibitor class has lowered the "cost-per-patient-month" by an average of 40% across Europe and North America.
Investment Milestone: R&D spending on ADCs by major pharmaceutical companies (Pfizer, AstraZeneca) surged by 20% in 2025, targeting "hard-to-treat" solid tumors.
Pipeline Indicator: There are currently over 800 biologics in Phase II and III trials globally, with a significant portion dedicated to personalized medicine and rare diseases.
Biosimilars and Biologics Market Regional Insights:-
North America
Market Share: 42.8% of the global biosimilars & biologics market, led by the United States with strong regulatory support (FDA), high adoption of biosimilars, and advanced healthcare infrastructure.
Insight: North America dominates due to mature regulatory pathways, rapid uptake in oncology and chronic disease biosimilars, and significant presence of major biotech/pharma firms.
Europe
Market Share: 38% of global biosimilars & biologics market, driven by early adoption and supportive regulatory frameworks like the EMA.
Insight: Europe remains highly mature in biosimilar adoption with strong competitive markets in Germany, the UK, and France; cost-containment initiatives further support uptake.
Asia Pacific
Market Share: 30% and fastest growth region, underpinned by expanding healthcare access, rising prevalence of chronic diseases, and growing biosimilars production in China and India.
Insight: Asia Pacific's share is lower than North America and Europe today but is growing quickly due to increasing demand for cost-effective biologics and investments in local manufacturing.
Biosimilars and Biologics Market Market Segmentation
By Type:
The Biosimilars and Biologics market is segmented into biologics and biosimilars. Biologics are complex, large-molecule drugs derived from living cells and are widely used in treating chronic and life-threatening diseases due to their high specificity and effectiveness. Biosimilars, on the other hand, are highly similar versions of approved biologic drugs, offering comparable safety and efficacy at a lower cost, thereby improving treatment accessibility and driving market competition.
By Application:
Based on application, the market covers oncology, autoimmune diseases, chronic diseases, infectious diseases, neurology, ophthalmology, and others. Oncology holds a significant share due to the rising global cancer burden and increasing demand for targeted therapies. Growing prevalence of autoimmune and chronic diseases further boosts demand for biologics and biosimilars, while expanding research in neurology and ophthalmology continues to create new growth opportunities in advanced therapeutic treatments.
Need more?
Speak to our analyst to understand how this research was put together
Add more segments or countries to the scope as part of free customization
Understand how this report can have a direct impact on your revenue
Get Customization in the report as per your requirements:https://www.datamintelligence.com/customize/biosimilars-and-biologics-market?prtk
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilars and Biologics Market is projected to reach US$ 1.77 trillion by 2033, growing at a CAGR of 14.4%, North America is expected to dominate the market with a 42.8% share here
News-ID: 4386273 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
Heterogeneous Integration Market to Reach US$ 10.2 Billion by 2031 at 35.7% CAGR …
The heterogeneous integration market reached US$ 0.9 billion in 2023 and is projected to surge to US$ 10.2 billion by 2031, expanding at an exceptional CAGR of 35.7% during the forecast period from 2024 to 2031. Heterogeneous integration refers to the advanced semiconductor approach of combining multiple chip components such as CPUs, GPUs, memory, and specialized accelerators into a single package to deliver superior computing performance, improved power efficiency, and…

Natural Gas Engine Market Expected to Reach USD 8.97 Billion by 2032, with Asia …
The global Natural Gas Engine Market reached US$5.34 billion in 2023, rising to US$5.66 billion in 2024 and is expected to reach US$8.97 billion by 2032, growing at a strong CAGR of 5.9% during the forecast period from 2025 to 2032.
The Natural Gas Engine Market is expanding as stricter emissions regulations and rising demand for cleaner, cost-effective power solutions drive adoption across transport and industrial sectors, supported by expanding natural…
Mammography market share is influenced by screening adoption, and the competitiv …
The Global Mammography Market reached USD 2.5 billion in 2022 and is projected to reach USD 5.3 billion by 2031, growing at a CAGR of 10.1% during the forecast period 2024-2031.
DataM Intelligence unveils exclusive insights into the Global Mammography Market 2026, highlighting technological advancements in breast imaging, rising breast cancer screening programs, and increasing adoption of digital and 3D mammography systems across healthcare facilities worldwide.
Download your exclusive sample report today…

Ultrasound Devices Market is projected to reach USD 13.07 billion by 2030, led b …
The global Ultrasound Devices Market size was estimated at USD 9.79 billion in 2023 and is anticipated to reach USD 13.07 billion by 2030, growing at a CAGR of 4.24% from 2024 to 2030. The market growth is poised to be driven by the rising usage of ultrasound equipment for diagnostic imaging and treatment, along with the increasing prevalence of chronic and lifestyle disorders.
Get a Free Sample PDF Of This…
More Releases for Biologics
Global Biologics Contract Manufacturing Market Set to Reach $83.47 Billion by 20 …
Biologics Contract Manufacturing Market reached US$ 26.03 billion in 2024 and is expected to reach US$ 83.47 billion by 2033, growing at a CAGR of 14.0% during the forecast period 2025-2033.
The Biologics Contract Manufacturing market involves outsourcing the production of complex biologic drugs, including vaccines, antibodies, and recombinant proteins. Rising demand for biologics, cost-effective manufacturing, and scalable production drives growth. Technological advancements in cell culture, purification, and quality control enhance…
Advanced Biologics Manufacturing Solutions Market Future Business Opportunities …
The qualitative latest Research report (2025-2032) on the Advanced Biologics Manufacturing Solutions Market by Coherent Market Insights Provides a deep dive into key market trends, drivers, challenges, and the competitive landscape. It analyzes market size, revenue, production, and CAGR using validated methodologies to ensure precision. The report highlights tech innovation, pricing trends, consumer behavior, and investment potential - empowering businesses to make informed, strategic moves.
Request a Sample Copy: https://www.coherentmarketresearch.com/samplepages/137417
Focused on…
Biologics Fill Finish Manufacturing Market 2024-2031: Global Industry Size, Shar …
According to a new report published by CoherentMI The Biologics Fill Finish Manufacturing Market is estimated to be valued at USD 5.41 Billion in 2024 and is expected to reach USD 9.04 Billion by 2031, growing at a compound annual growth rate (CAGR) of 7.5% from 2024 to 2031.
Most recent Report, named "Biologics Fill Finish Manufacturing Market" Patterns, Offer, Size, Development, Opportunity and Forecast 2024-2031, by CoherentMI offers a…
Biologics Market
The "Biologics Market" is expected to reach USD xx.x billion by 2031, indicating a compound annual growth rate (CAGR) of xx.x percent from 2024 to 2031. The market was valued at USD xx.x billion In 2023.
Growing Demand and Growth Potential in the Global Biologics Market, 2024-2031
Verified Market Research's most recent report, "Biologics Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2030," provides an in-depth examination of the industry…
Biologics Contract Manufacturing Market to Witness Huge Growth by 2031 | Wuxi Bi …
The Biologics Contract Manufacturing Market study by DataM Intelligence offer an in-depth analysis of the market, presenting insightful observations, statistics, historical data, and industry-validated market insights. The report delves into the competitive positioning of key companies, examining factors such as product offerings, pricing strategies, financial health, product portfolios, growth initiatives, and geographical reach.
Download a Free sample PDF (Use Corporate email ID to Get Higher Priority) at: - https://datamintelligence.com/download-sample/biologics-contract-manufacturing-market
What is the…
Spine Biologics Market Report 2024 - Spine Biologics Market Trends And Share
"The Business Research Company recently released a comprehensive report on the Global Spine Biologics Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive Sample…