Press release
The Contract Development and Manufacturing Market size is expected to reach US$ 280.07 billion by 2033, dominated by North America with a 45% market share
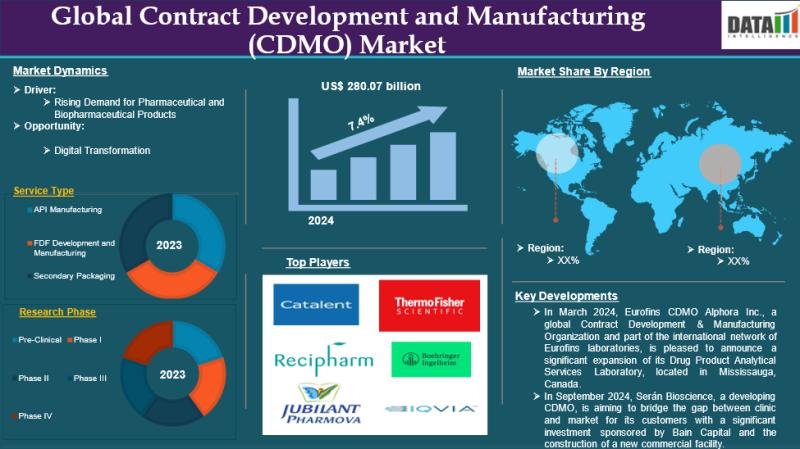
The global Contract Development and Manufacturing (CDMO) Market reached US$ 150.19 billion in 2024 and is expected to reach US$ 280.07 billion by 2033, growing at a CAGR of 7.4% during the forecast period 2025-2033.Rising demand for innovative biologics, small molecules, and high-complexity therapies, coupled with increasing R&D spending and technological advancements in continuous manufacturing, automation, and quality control, is expanding the CDMO market globally.Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/contract-development-and-manufacturing-market?prtk
United States: Key Industry Developments (CDMO Market 2026 & 2025)
✅ January 2026: Thermo Fisher Scientific secured multiple major CDMO contracts to help pharmaceutical clients shift production back to the U.S., supporting reshoring of drug manufacturing and expanding domestic contract services.
✅ January 2026: Lonza announced its outlook for 2026 with projected double-digit sales growth (11.5%) in its core CDMO business, driven by ongoing strong demand at its Vacaville, California biologics and small-molecule manufacturing facilities.
✅ February 2026: The U.S. FDA launched the PreCheck pilot program to expedite pharmaceutical manufacturing facility reviews and construction, designed to accelerate CDMO-related plant development and quality systems alignment in the U.S. market.
✅ December 2025: Samsung Biologics completed acquisition of a U.S. contract drug production facility in Maryland and plans further capacity investments to enhance its CDMO footprint and technology offerings in the country.
Japan: Key Industry Developments (CDMO Market 2026 & 2025)
✅ December 2025: FUJIFILM Biotechnologies completed a large-scale antibody drug CDMO facility in Toyama, Japan, equipped with 10,000 L bioreactor capacity creating a new biologics manufacturing hub to serve domestic and Asia-Pacific clients.
✅ December 2025: Japan's government allocated ¥15.8 billion ($100 M) to support regenerative medicine and cell/gene therapy manufacturing expansion, bolstering CDMO capacity and advanced bioprocessing tech adoption.
✅ October 2025: Cell Therapies and Teijin formed a strategic CDMO partnership to expand access to advanced cell and gene therapy manufacturing in Japan and the Asia-Pacific, strengthening regional GMP capabilities.
✅ September 2025: Nikon CeLL Innovation committed over ¥10 billion ($68 M) to expand its regenerative medicine CDMO facilities in Tokyo, increasing floor space and capabilities for CAR-T, iPS, and MSC manufacturing.
Contract Development and Manufacturing Market Recent M&A activities:-
→ On January 15, 2026, Zydus Lifesciences Limited completed the acquisition of two biologics contract development and manufacturing facilities from Agenus Inc. (located in Emeryville and Berkeley, California, US). The transaction included US $75 million upfront cash, US $16 million equity investment, and up to US $50 million of contingent milestone payments, bringing the total consideration to up to approximately US $141 million as part of a broader strategic collaboration to scale global biologics manufacturing and commercialization of key immuno-oncology assets. This transaction establishes Zydus' presence in the global CDMO business under its U.S. subsidiary Zylidac Bio LLC.
→ On November 7, 2025, LTS LOHMANN Therapie-Systeme AG completed its acquisition of Renaissance Lakewood, LLC, a U.S.-based contract development and manufacturing organization (CDMO) specialized in nasal sprays and sterile dosage forms. Financial terms were not publicly disclosed. The deal significantly expands LTS' global CDMO capabilities and integrates Renaissance into its network as a dedicated division.
Contract Development and Manufacturing Market key Players:-
Catalent Inc, Recipharm AB, Jubilant Pharmova Limited, Thermo Fisher Scientific Inc., Boehringer Ingelheim International GmbH, IQVIA, Syneos Health, Parexel International (MA) Corporation, Curia Global, Inc., NextPharma Technologies among others.
Top 5 Key Players Analysis:-
1. Catalent Inc.
Catalent holds around a 16.3% share in the CDMO market, making it a leading global provider with strong biologics and oral dosage capabilities.
Its strengths lie in end-to-end development and manufacturing for biologics, gene therapies, and commercial drug products across multiple global sites.
2. Thermo Fisher Scientific Inc.
Thermo Fisher has about a 15.4% market share, leveraging its vast scale and integrated pharma services platform.
It excels in API development, biologics production, and commercial manufacturing solutions for both large pharma and biotech.
3. Recipharm AB
Recipharm captures approximately 12.8% of the market with a strong European manufacturing footprint.
Its core strengths include expertise in injectables, inhalation, and oral solid dosage formulations with growing sterile operations.
4. Boehringer Ingelheim International GmbH
Boehringer Ingelheim holds roughly 10.2% share, driven by its large-scale biopharmaceutical manufacturing under the BioXcellence brand.
The company's strengths include microbial and biologics production with a focus on scalable commercial manufacturing.
Contract Development and Manufacturing Market Top Technological Partnerships (2026 & 2025):-
AI-Powered Small Molecule Optimization: In February 2026, Lonza entered a strategic technology partnership with a leading AI drug-discovery firm to integrate machine learning into its "Route Scouting" services. This collaboration aims to predict the most efficient chemical synthesis pathways for complex small molecules, potentially reducing early-stage development timelines by 25%.
Next-Gen ADC Manufacturing Cluster: In January 2026, WuXi Biologics and WuXi STA deepened their "WuXi XDC" collaboration through a joint technology venture focused on one-stop Antibody-Drug Conjugate (ADC) development. The partnership introduces a proprietary linker-payload technology that simplifies the conjugation process, allowing biopharma clients to move from DNA sequence to Investigational New Drug (IND) filing in under 15 months.
Scalable mRNA Production Hub: In December 2025, Samsung Biologics partnered with a specialized biotech firm to implement an end-to-end mRNA manufacturing workflow at its Songdo facility. This partnership utilizes advanced lipid nanoparticle (LNP) encapsulation technology, designed to provide high-stability delivery systems for next-generation vaccines and oncology therapeutics.
Automated Cell Therapy Processing: In November 2025, Thermo Fisher Scientific announced a technical partnership with a robotics specialist to deploy fully automated, closed-loop cell therapy manufacturing systems. This "Factory of the Future" initiative aims to reduce human touchpoints in CAR-T cell production, lowering the risk of contamination and decreasing per-patient manufacturing costs by an estimated 30%.
Biocatalysis for Green Chemistry: In October 2025, Eurofins CDMO teamed up with an enzyme engineering company to integrate biocatalysis into its API (Active Pharmaceutical Ingredient) manufacturing. This partnership focuses on replacing heavy-metal catalysts with tailored enzymes, promoting "Green Chemistry" standards that significantly reduce the environmental footprint of large-scale chemical synthesis.
High-Potency API (HPAPI) Safety Standards: In September 2025, Recipharm partnered with a safety technology provider to implement advanced isolator and containment systems for High-Potency API manufacturing. The collaboration focuses on enhancing safety protocols for the production of highly toxic oncology drugs, ensuring zero-exposure environments for operators while maintaining high throughput.
This report addresses:
Market intelligence to enable effective decision making
Market estimates and forecasts from 2024-2032
Growth opportunities and trend analyses
Segment and regional revenue forecasts for market assessment
Competition strategy and market share analysis
Product innovation listing for you to stay ahead of the curve
COVID-19's impact and how to sustain in these fast-evolving markets
Buy Now & Unlock 360° Market Intelligence:https://www.datamintelligence.com/buy-now-page?report=contract-development-and-manufacturing-market?prtk
Contract Development and Manufacturing Market Drivers :-
Expansion Figure: Specialized biologics now account for nearly 50% of the R&D pipeline, leading to a 14% CAGR in the biologics CDMO segment through 2026.
Efficiency Gain: By utilizing standardized "plug-and-play" cleanroom modules, CDMOs have reduced the time to set up new cell therapy production lines from 18 months to just 6 months.
Market Indicator: CDMOs in North America, Europe, and India have reported a 20-30% increase in inquiries from major biopharma companies looking to relocate critical manufacturing assets as of late 2025.
Investment Milestone: Over $15 billion in new capacity investments were announced in 2025 for "Western-based" manufacturing hubs to ensure supply chain security for essential medicines.
Measurable Impact: AI-integrated "digital twins" of manufacturing processes have demonstrated the ability to improve yield by 15-20% while reducing batch failure rates by nearly 30%.
Adoption Rate: As of early 2026, 4 out of the top 5 global CDMOs have implemented AI-based predictive maintenance across their primary manufacturing sites.
Growth Indicator: Outsourcing rates among "Emerging Biopharma" companies have hit a record high of 80%, as these firms lack the capital to build their own multi-million dollar manufacturing plants.
Transaction Volume: New contract signings for Phase I and Phase II clinical trial materials increased by 12% year-over-year in the 2025 fiscal year.
Efficiency Milestone: Leading CDMOs have achieved a 25% reduction in solvent waste through the adoption of "Continuous Manufacturing" technology, which replaces traditional, waste-heavy batch processing.
Market Pressure: By 2026, an estimated 70% of RFPs (Request for Proposals) from Top 20 Pharma companies include mandatory ESG (Environmental, Social, and Governance) disclosure and reduction targets.
Contract Development and Manufacturing Market Regional Insights:-
North America
North America accounts for the largest share of the global contract development and manufacturing market (45% in pharmaceutical CDMO segments), led by a mature biotech/pharma ecosystem and strong regulatory infrastructure.
This region's dominance is driven by U.S. pharmaceutical R&D spending, extensive biologics outsourcing, and high adoption of advanced manufacturing services by leading pharma companies.
Europe
Europe holds roughly 32% of the global CDMO/contract manufacturing market, with key hubs in Germany, Switzerland, and Ireland benefiting from strong quality standards and a mature industry base.
The region's market is supported by established pharmaceutical manufacturing capacities and increasing investment in biologics and specialty services.
Asia-Pacific
Asia-Pacific represents 27% (pharma CDMO) of the global market and is often the fastest-growing region due to cost-effective manufacturing, expanding biotech investments, and rising outsourcing from global pharma firms.
Countries like China and India are major contributors, leveraging lower costs and increasing regulatory alignment with global quality standards.
Contract Development and Manufacturing Market Market Segmentation
By Service Type:
The CDMO market is segmented by service type into API manufacturing, finished dosage formulation (FDF) development and manufacturing, and secondary packaging. API manufacturing focuses on producing active drug ingredients, while FDF covers formulation, processing, and final drug production. Secondary packaging includes labeling, serialization, and compliance packaging, ensuring regulatory adherence and product safety.
By Research Phase:
Based on research phase, the market covers pre-clinical through Phase I-IV stages. CDMOs support drug development from early laboratory testing to large-scale clinical trials and post-marketing studies. Demand increases in later phases due to larger production volumes, regulatory validation, and commercialization support.
By End User:
By end user, the CDMO market serves pharmaceutical companies, contract research organizations (CROs), and generic drug manufacturers. Pharmaceutical companies outsource to reduce costs and accelerate timelines, CROs collaborate for integrated development solutions, and generic manufacturers rely on CDMOs for cost-efficient, high-volume production.
Need more?
Speak to our analyst to understand how this research was put together
Add more segments or countries to the scope as part of free customization
Understand how this report can have a direct impact on your revenue
Get Customization in the report as per your requirements:https://www.datamintelligence.com/customize/contract-development-and-manufacturing-market?prtk
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The Contract Development and Manufacturing Market size is expected to reach US$ 280.07 billion by 2033, dominated by North America with a 45% market share here
News-ID: 4386179 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
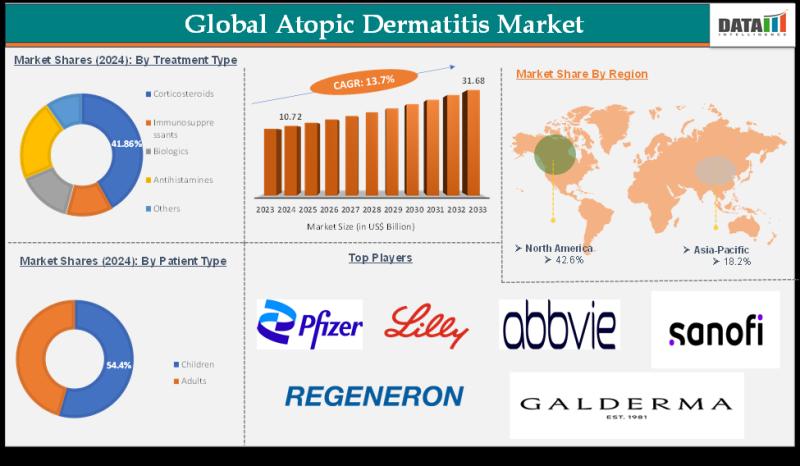
Atopic Dermatitis Market to hit $31.68 billion by 2033 | Major Market Players 20 …
The Atopic Dermatitis Market reached US$ 10.72 billion in 2024 and is expected to reach US$ 31.68 billion by 2033, growing at a CAGR of 13.7% during the forecast period 2025-2033.
DataM Intelligence unveils exclusive insights into the Global Atopic Dermatitis Market 2026, highlighting accelerated innovation in biologics and small molecules, expanding treatment access across age groups, and strong pipeline activity among dermatology-focused biopharmaceutical companies.
Atopic dermatitis (AD) is a chronic, relapsing…
Regenerative Medicine Market is projected to reach US$403.86 billion by 2032, gr …
The global Regenerative Medicine Market reached US$ 48.45 billion in 2024 and is expected to reach US$ 403.86 billion by 2032, growing at a CAGR of 27.3 % during the forecast period 2025-2033.
The Regenerative Medicine market is expanding rapidly due to rising chronic and degenerative diseases, extensive R&D in stem cell and gene therapies, and advanced biotechnologies like 3D bioprinting that enable personalized and restorative treatments, improving patient outcomes globally.
Get…
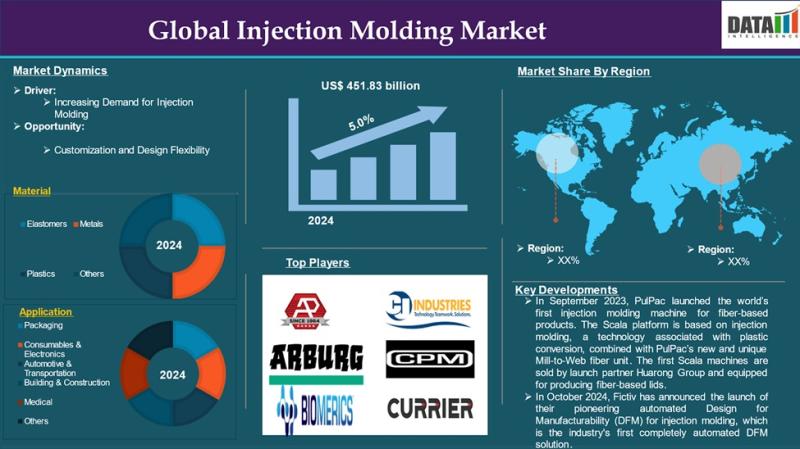
Injection Molding Market to Reach US$ 451.83 Billion by 2033 at 5% CAGR | North …
The injection molding market reached US$ 295 billion in 2024 and is projected to grow to US$ 451.83 billion by 2033, registering a CAGR of 5% during the forecast period from 2025 to 2033. Injection molding is a highly efficient manufacturing process in which molten material such as plastics, metals, glass, or elastomers is injected into precision molds to create complex and uniform components at scale. Its ability to support…

ASEAN Semiconductor Market Set for Steady Growth by 2031, Led by Asia Pacific's …
ASEAN Semiconductor Market growth is driven by the rising demand for advanced chips across automotive, consumer electronics, and industrial automation sectors, alongside accelerating digital transformation across Southeast Asia.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/custom-research?kb
United States: Recent Developments
✅ January 2026: U.S. semiconductor firms announced new supply chain partnerships with ASEAN foundries to boost regional production resilience.
Japan: Recent Developments
✅ January 2026: Japanese semiconductor…
More Releases for CDMO
FDP CDMO Research: China FDP CDMO market size is projected to reach USD 1.33 bil …
QY Research Inc. (Global Market Report Research Publisher) announces the release of 2025 latest report "Fraud Detection and Prevention (FDP) System- Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031". Based on current situation and impact historical analysis (2020-2024) and forecast calculations (2025-2031), this report provides a comprehensive analysis of the global Wire Drawing Dies market, including market size, share, demand, industry development status, and forecasts for the…
Global Cmo And Cdmo Biotechnology Market Size by Application, Type, and Geograph …
According to Market Research Intellect, the global Cmo And Cdmo Biotechnology market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
Biologics and sophisticated medicines are driving the biotechnology industry for Contract Manufacturing Organizations (CMO) and Contract…
Evolving Market Trends In The Inhalation CDMO Industry: Strategic Collaborations …
The Inhalation CDMO Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Inhalation CDMO Market Size During the Forecast Period?
In recent times, the inhalation CDMO market has experienced significant growth. The market value is expected to increase from $2.08 billion in 2024…
What's Driving the Inhalation CDMO Market 2025-2034: Rising Respiratory Disorder …
How Is the Chondroplasty Market Projected to Grow, and What Is Its Market Size?
The chondroplasty market has seen strong growth in recent years. It will increase from $13.77 billion in 2024 to $14.68 billion in 2025 at a CAGR of 6.5%. This growth is attributed to the rise in sports-related injuries, patient preference for non-total joint replacement procedures, advances in postoperative care, healthcare provider training, and an increasing incidence of…
Lentiviral Vector (LVV) CDMO Services Market Delivering Cures: The Role of LVV C …
Lentiviral Vector (LVV) CDMO Services Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Lentiviral Vector (LVV) CDMO Services Market - (By Type (IIT Grade, IND Grade, Clinical Trial Grade, Commercial Production Grade), By Application (Biopharmaceutical Company, Academic Scientific Research Institution)), Trends, Industry Competition Analysis, Revenue and Forecast To…
Electronic Chemicals CDMO Market Fueling the Electronics Boom: The Rise of the E …
Electronic Chemicals CDMO Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Electronic Chemicals CDMO Market - (By Type (Metals and Pastes, Electronic Specialty Gases, Polymer Compounds, Others), By Application (Battery, Semiconductor, Integrated Circuit, Consumer Electronics, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest…