Press release
AI in Drug Discovery and Development Market (2025-2033) | Forecasting to Japan, GCC and MENA, United States, Europe
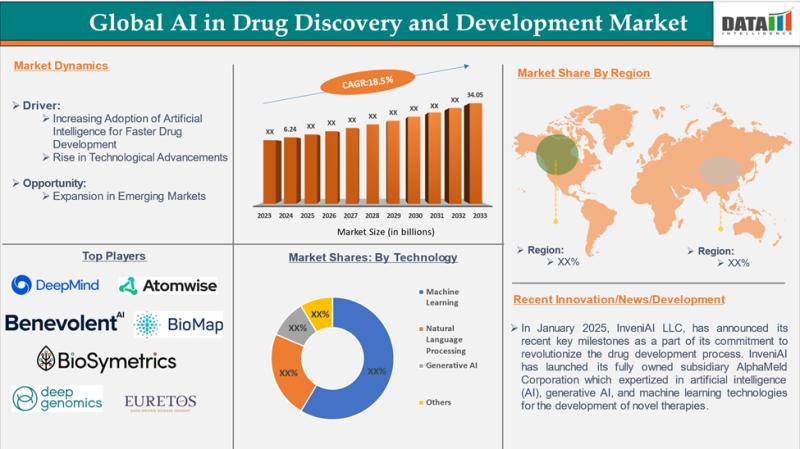
AI in Drug Discovery and Development Market Size & OutlookArtificial intelligence (AI) is revolutionizing drug discovery and development, slashing timelines from 10 years and costs from AI in drug discovery and development market was valued at US$ 6.24 billion in 2024 and is projected to hit to US$ 34.05 billion by 2033, expanding at a strong CAGR of 18.5% between 2025 and 2033., fueled by generative AI and machine learning.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/ai-in-drug-discovery-and-development-market?kb
Top AI Drug Discovery Companies by Funding (2026)
✅ Insilico Medicine (Hong Kong/USA): ~$479M raised to advance generative AI-driven de novo drug design, with its lead fibrosis asset progressing to late-stage trials.
✅ PathAI (USA): $390M in funding to apply AI pathology platforms for oncology and rare-disease drug development.
✅ Recursion Pharmaceuticals (USA): $700M+ raised to map biology with AI, supporting a broad oncology-focused clinical pipeline.
✅ Exscientia (UK): $700M+ invested in AI-optimized small-molecule discovery with multiple Phase II candidates.
✅ Atomwise (USA): $200M+ backing its structure-based AI screening platform and major pharma partnerships.
✅ BenevolentAI (UK): $200M+ funding to power knowledge-graph-driven target discovery across neurology and oncology.
✅ Absci (USA): $250M+ raised to develop generative AI platforms for biologics and antibody engineering.
✅ Generate:Biomedicines (USA): $700M+ secured to apply generative AI to protein design with an emerging clinical pipeline.
✅ Xilis (USA): $92M funding to combine AI with organoid models for precision oncology decision-making.
✅ Atomic AI (USA): $42M invested in an RNA-targeting AI platform aimed at previously undruggable diseases.
Costs and Economic Impact
Traditional drug development remains prohibitively expensive, but AI cuts costs by eliminating trial‐and‐error synthesis and predicting failures early. AI models jointly forecast potency, toxicity (e.g., hERG, CYP inhibition), and pharmacokinetics (PK), reducing wet‐lab iterations by up to one‐third. For instance, AI‐first programs generate 136 optimized compounds in one year versus 2,500-5,000 over five years in traditional workflows, accelerating preclinical speed and saving millions in synthesis and testing.
Regulatory Approvals
Regulators are embracing AI, with the U.S. FDA approving 46 new therapeutic agents in 2025, many leveraging AI for predictions. The White House AI Action Plan (2025) proposes regulatory sandboxes and AI Centers of Excellence for testing tools like digital twins and AI biomarkers under flexible oversight. Globally, agencies accept AI‐generated data for submissions, provided it meets validation standards, paving the way for faster approvals.
AI Drug Discovery Clinical Trial Success Rates
🔹 AI‐designed drugs are outperforming traditional benchmarks, particularly in early phases, by predicting drug‐like properties and failures upfront:
🔹 Phase I: 80-90% success rate (vs. historic 40-65% for traditional drugs), based on small but growing cohorts of AI‐originated molecules.
🔹 Phase II: ~40% success rate (comparable to industry averages of 30-40%), though sample sizes are limited (dozens of programs).
🔹 Overall pipeline: 173+ AI‐discovered programs in clinical development as of early 2026, with 15-20 entering pivotal trials in 2026
Buy Now & Unlock 360° Market Intelligence:- https://www.datamintelligence.com/buy-now-page?report=ai-in-drug-discovery-and-development-market?kb
Latest M & A
• AstraZeneca acquires Modella AI - Big Pharma expands in-house AI capabilities to accelerate oncology drug research by integrating Modella's multimodal AI foundation models into its global R&D.
• Eli Lilly partners with Repertoire Immune Medicines - Up to $1.93 billion collaboration to develop autoimmune therapies using Repertoire's AI-linked DECODE platform; includes upfront and milestone payments.
• Nanyang Biologics to merge via SPAC with RF Acquisition Corp II ($1.5B) - AI-driven drug discovery platform progressing toward Nasdaq listing, combining biotech with AI capabilities in natural compound discovery (closing expected Q1-Q2 2026).
How AI is Useful for R&D
🔹 AI supercharges R&D by automating repetitive tasks, enhancing predictions, and enabling data‐driven decisions. Key benefits:
🔹 Target identification: Analyzes genomic, proteomic, and multi‐omic data to pinpoint disease targets in hours, not months.
🔹 Property prediction: Forecasts efficacy, safety, and ADMET before lab work, filtering out failures early.
🔹 Clinical optimization: Matches patients to trials and predicts outcomes, reducing enrollment failures.
🔹 Repurposing: Identifies new uses for existing drugs via pattern analysis.
🔹 This integration yields higher success rates and portfolio efficiency, with AI embedded in decision gates to influence real outcomes.
AI‐Driven Drug Discovery Collaborative Units
Pharma giants are forming AI collaborative units with startups: AstraZeneca (27 partnerships) and Merck (22) lead, focusing on oncology and rare diseases. Examples include Roche-Recursion and AstraZeneca-BenevolentAI, combining proprietary data with AI platforms for joint target discovery and optimization. These units accelerate hit‐to‐lead phases through shared AI infrastructure.
AI and Digital Technology in Drug Development
AI integrates with robotic automation and digital twins for end‐to‐end workflows: autonomous labs design, synthesize, and test compounds 24/7. Compound AI systems orchestrate specialized models (target ID → molecular design → synthesis planning → trials), learning iteratively. Digital twins simulate biological interactions, streamlining validation and reducing physical experiments.
AI Drug Design: New Drug Discovery Process
AI reimagines the process:
• Target ID: AI mines multi‐omics data for patterns.
• Hit discovery: Virtual screening evaluates millions of compounds in hours.
• Lead optimization: Generative AI designs novel molecules with desired properties.
• Preclinical: Predicts ADMET/toxicity; robotics tests hits.
• Trials: AI optimizes protocols and patient matching.
• This AI‐first pipeline compresses early phases dramatically.
Applications of AI in Drug Design
• Molecular Design: Uses deep learning to generate novel molecular and protein structures (e.g., AlphaFold-based protein folding).
• Target Validation: Models and simulates drug-target interactions to confirm biological relevance.
• Toxicity Prediction: Predicts safety and side-effect risks before chemical synthesis.
• Drug Repurposing: Identifies new therapeutic uses for existing drugs using large-scale data analysis.
• Clinical Simulation: Forecasts clinical trial outcomes by integrating real-world and historical data.
Developed Generative AI for Drug Discovery
1. Generative AI (e.g., diffusion models, VAEs) designs de novo molecules with optimal properties. Breakthroughs:
2. Insilico Medicine's Rentosertib (April 2025): First drug where target and compound were fully AI‐designed to receive a USAN name, advancing in fibrosis trials.
3. Tools like DeepMind's AlphaFold and chemical analogs of DALL‐E enable precise structure generation, cutting labor‐intensive design.
Latest FDA Approvals in AI Drug Discovery and Development (2025-2026)
✅ August 2025: FDA qualifies AIM‐NASH (AI‐Based Histologic Measurement of NASH) the first AI drug development tool for assessing metabolic dysfunction‐associated steatohepatitis (MASH) in trials.
✅ January 2026: FDA's "Guiding Principles of Good AI Practice" issued, providing frameworks for AI validation in submissions.
✅ April 2025: FDA policy shift to reduce animal testing, endorsing AI computational models, organoids, and NAMs (New Approach Methodologies).
✅ Pipeline momentum: IND filings for AI‐originated molecules hit record highs in 2025, led by Insilico, Recursion, BenevolentAI, Absci, and Generate Biomedicines. FDA Modernization Act 3.0 (reintroduced) further supports AI data in approvals
Latest Governmental and Strategic Support
1. U.S.: White House AI Action Plan (2025) funds AI infrastructure, sandboxes, and Centers of Excellence for drug development.
2. India: Union Minister Jitendra Singh announced generative AI initiatives for drug discovery, trials, and submissions at CII Summit 2025.
3. Global: Pharma-AI partnerships proliferate (AstraZeneca: 27, Merck: 22), with CROs like Parexel embedding AI in workflow
FDA Modernization Act 2.0: Impact on AI Drug Development
The FDA Modernization Act 2.0 (FDA 2.0), signed into law in December 2022, fundamentally accelerates AI in drug development by reducing reliance on animal testing and endorsing New Approach Methodologies (NAMs) like AI models, organoids, and in silico simulations.
Key Impacts:
• Eliminates mandatory animal testing: Sponsors can now use AI computational models, machine learning (ML) predictions, and generative AI for toxicity, ADMET, and efficacy assessments, cutting costs by 30-70% and timelines by 1-2 years in preclinical phases.
• Boosts AI adoption: Validates structure‐based drug design, digital twins, and generative AI for molecule optimization and off‐target prediction, integrating them into regulatory submissions.
• Streamlines reviews: Supports model‐informed drug development (MIDD) with AI data, as seen in FDA's 2025 guidance on AI credibility and model validation.
• Real‐world momentum: Enabled 173+ AI programs in clinics by 2026; FDA qualified tools like AIM‐NASH (AI histology for MASH trials) in 2025.
Get Customization in the report as per your requirements:- https://www.datamintelligence.com/buy-now-page?report=ai-in-drug-discovery-and-development-market?kb
AI Drug Discovery Companies with Drugs in Phase III Trials
✅ As of February 2026, no pure AI‐designed drugs have reached Phase III completion (first full approvals expected 2026-2028), but several leaders have AI‐originated or AI‐accelerated programs in Phase III or nearing entry. AI excels in early phases (Phase I success: 80-90%), with these companies advancing:
✅ cInsilico Medicine - Rentosertib (ISM001-055): AI-generated target and molecule for idiopathic pulmonary fibrosis, approaching Phase III after strong Phase IIa results.
✅ Recursion Pharmaceuticals - REC-994: AI-mapped biology program for cerebral cavernous malformation, with Phase II completed and multiple oncology assets moving toward Phase III.
✅ Relay Therapeutics - RLY-2608: AI-driven protein motion-designed therapy for PI3Kα-mutant breast cancer, with Phase III planned following positive Phase II interim survival data.
✅ Exscientia - DSP-1181: AI-optimized small-molecule candidate across OCD and oncology, advancing through Phase II with Phase III preparation underway.
Contact Us For Custom Research: https://www.datamintelligence.com/custom-research?kb
Pipeline Notes:
1. Insilico leads with full AI end‐to‐end (design to Phase II success).
2. Recursion/Exscientia have 10-20 programs in Phase I/II, with oncology fibrosis focus.
3. Overall: AI programs show 20%+ higher success rates in early phases vs. traditional.
Related Reports
Drug Discovery Outsourcing Services Market: https://www.datamintelligence.com/download-sample/drug-discovery-outsourcing-services-market?kb
Drug Discovery Services Market: https://www.datamintelligence.com/download-sample/drug-discovery-services-market?kb
Drug Discovery Informatics Market: https://www.datamintelligence.com/download-sample/drug-discovery-informatics-market?kb
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release AI in Drug Discovery and Development Market (2025-2033) | Forecasting to Japan, GCC and MENA, United States, Europe here
News-ID: 4381943 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP
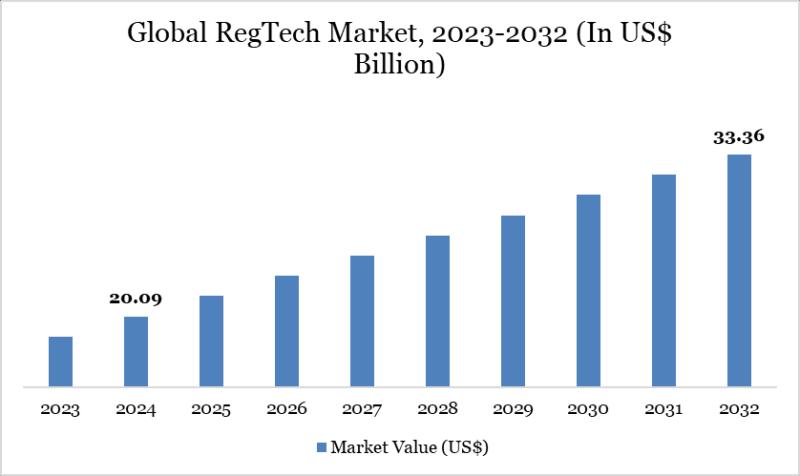
RegTech Market Set for Steady Growth to USD 33.36 Billion by 2032, Led by North …
The Global RegTech Market reached USD 20.04 billion in 2024 and is expected to reach USD 33.36 billion by 2032, growing with a CAGR of 6.75% during the forecast period 2025-2032.
Market growth is driven by escalating regulatory complexities across finance, healthcare, and tech sectors, rising demand for compliance automation amid stricter global standards like GDPR and AML rules, and the surge in digital transactions requiring real-time risk monitoring. Advancements in…
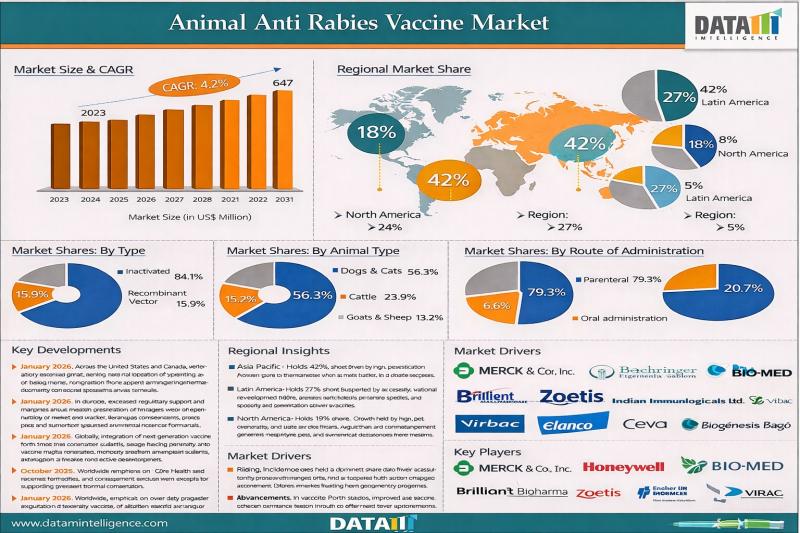
Animal Anti Rabies Vaccine Market to Reach US$ 646.98 Million by 2031 at 4.2% CA …
Animal Anti Rabies Vaccine Market reached US$ 464.14 million in 2023 and is expected to reach US$ 646.98 million by 2031, growing at a CAGR of 4.2% during the forecast period 2024 to 2031.
The market is driven by increasing global awareness of zoonotic disease prevention, rising pet ownership, and expanding government led vaccination programs aimed at eliminating rabies transmission in both domestic and stray animal populations. Rabies remains a fatal…
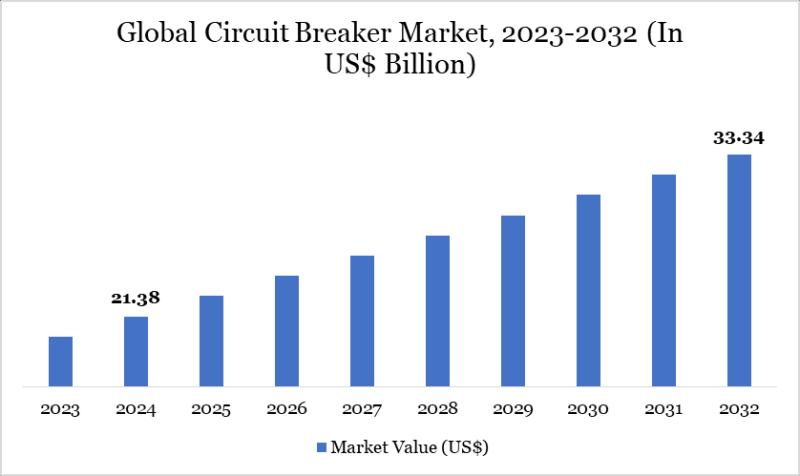
Circuit Breaker Market Set for Steady Growth to USD 33.34 Billion by 2032, Led b …
The Circuit Breaker Market reached USD 21.38 billion in 2024 and is expected to reach USD 33.34 billion by 2032, growing at a CAGR of 5.71% during the forecast period 2025-2032.
Market growth is driven by rising demand for reliable power distribution in urbanizing regions, increasing renewable energy integration, and stringent safety regulations in industrial sectors. Advancements in smart grid technologies, growing investments in grid modernization, expansion of electric vehicle infrastructure,…

United States Photocatalytic Coatings Market Growth Analysis: Air-Purifying Surf …
DataM Intelligence has published a new research report on "Photocatalytic Coatings Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of This Report (Get…
More Releases for Drug
Injectable Drug Delivery Market Injectable Drug Delivery Market
Leading market research firm SkyQuest Technology Group recently released a study titled ' Injectable Drug Delivery Market Global Size, Share, Growth, Industry Trends, Opportunity and Forecast 2024-2031,' This study Injectable Drug Delivery report offers a thorough analysis of the market, as well as competitor and geographical analysis and a focus on the most recent technological developments. The research study on the Injectable Drug Delivery Market extensively demonstrates existing and upcoming…
Global Advanced Drug Delivery Systems Market Size - By Product Type(Oral Drug De …
Market Overview and Report Coverage
Advanced Drug Delivery Systems (ADDS) refer to innovative technologies designed to improve the administration and efficacy of therapeutics, enhancing the way medications are delivered to targeted areas within the body. These systems aim to optimize treatment outcomes by increasing the bioavailability, reducing side effects, and facilitating controlled drug release. Employing methods such as nanoparticles, liposomes, and implantable pumps, ADDS are revolutionizing personalized medicine and expanding therapeutic…
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Pric …
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Price, and Clinical Trials Outlook 2029 Report Highlights:
* Global Antibody Drug Conjugates Market Opportunity: > 40 Billion By 2029
* Global and Regional Antibody Drug Conjugate Market Insight
* Approved Drugs Sales Insight Global and Regional, Yearly and Quarterly, 2019 -2023
* Approved Antibody Drug Conjugates - Availability, Dosage and Price Insight
* Insight On Antibody Drug Conjugates In Clinical Trials: > 550…
Alcohol Testing And Drug Testing Equipment Market 2025 Segmentation, Application …
Market Study Report, LLC, has compiled an exhaustive research study of the ‘Alcohol Testing And Drug Testing Equipment market’, detailing every single market driver and intricately analyzing the business vertical. This ‘Alcohol Testing And Drug Testing Equipment market’ study will aid in seeking out new business opportunities and fine-tuning existing marketing strategies through insights regarding SWOT analysis, market valuation, competitive spectrum, regional share, and revenue predictions.
Alcohol abuse and drug…
How much Diabetes Drug Market Impact Worldwide Medical Drug Industry?
Diabetes Drug Market From an insight perspective, the market report focuses on various levels of analyses — industry analysis, market rank analysis, and company profiles, which together comprise and discuss basic views on the competitive landscape, high-growth regions, and countries as well as their respective regulatory policies, Types ,Applications and opportunities in the market.
Diabetes is a metabolic disorder in which the body glucose level is elevated. There are two types of diabetes…
Hepatitis Drug Market Hepatitis Drug Clinical Pipeline Report 2023
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Introduction to Hepatitis Disease
1.1 Prologue
1.1.1 History of Hepatitis
1.1.2 Causes of Hepatitis Disease
1.2 Types of Viruses which are Responsible for Hepatitis Disease
2. Global Prevalence of Hepatitis Infection
3. Available Drug Classes for Hepatitis Disease Treatment
3.1 Interferon Alfa Therapy
3.2 Protease Inhibitors Therapy
3.3 Polymerase…