Press release
Japan Drug Discovery Services Market Outlook (2026-2033): Key Developments and Investment Opportunities, Investment
The global drug discovery services market grew from USD 8.01 billion in 2023 to USD 8.37 billion in 2024 and is projected to expand further to USD 12.78 billion by 2033, registering a CAGR of 4.9% between 2025 and 2033.Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/drug-discovery-services-market?kb
Key Players
✅ Charles River Laboratories (USA): Delivers early-stage discovery support, including in-vivo/in-vitro studies and comprehensive safety assessments.
✅ Thermo Fisher Scientific (USA): Provides AI-enabled discovery platforms, high-throughput screening, and advanced bioanalytical solutions.
✅ Evotec SE (Germany): Offers fully integrated drug discovery programs across small molecules, biologics, CNS, and oncology.
✅ Labcorp Drug Development (USA): Supports discovery through preclinical development with biomarker-led and translational strategies.
✅ Pharmaron (China): Supplies end-to-end chemistry, biology, and preclinical services with a rapidly expanding global footprint.
Eurofins Discovery, WuXi AppTec, Syneos Health (Global): Specialize in screening, DMPK, and translational drug discovery services worldwide.
Capital Inflows and Evolving Supply Chains
• Investment momentum in drug discovery services remains strong, particularly for technology-driven platforms.
• Venture and private funding: AI-focused discovery companies and platform-based CROs attracted multi-billion-dollar investments during 2024-2025, reflecting confidence in data-driven target discovery and compound optimization.
• Pharma participation: Large pharmaceutical companies are increasingly backing discovery platforms through equity investments, co-development agreements, and licensing deals, especially in oncology, rare diseases, and metabolic disorders.
• Supply-chain optimization: Outsourcing chemistry, biology, and preclinical work to CROs and CDMOs is now standard practice, enabling faster execution and lower fixed costs.
• Resilience strategies: Post-pandemic supply-chain planning emphasizes regional diversification, dual sourcing, and reduced dependency on single geographies for key reagents and services.
Cost Dynamics in Drug Discovery
1• Drug discovery remains one of the most capital-intensive stages of pharmaceutical development.
2• In-house discovery economics: Developing a single approved drug internally can exceed USD 1-2 billion, driving companies toward outsourcing models.
3• Outsourced program costs: CRO-led discovery programs from hit identification to preclinical development typically range from USD 5 million to USD 50 million, depending on modality and complexity.
4• Geographic cost advantages: Regions such as India, China, and Eastern Europe offer cost savings of 20-40% compared with the U.S. and Western Europe, making them preferred hubs for early-stage chemistry and biology work.
Buy Now & Unlock 360° Market Intelligence:- https://www.datamintelligence.com/buy-now-page?report=drug-discovery-services-market?kb
Mergers, Acquisitions, and Industry Consolidation
✦ M&A activity in drug discovery services continues to accelerate as companies seek scale, technology access, and geographic reach.
✦ Pfizer-Seagen ($43B): Highlights strong demand for oncology assets emerging from late-stage, discovery-driven pipelines.
✦ Novo Holdings-Catalent ($16.5B): Enhances biologics development and manufacturing access closely aligned with early discovery programs.
✦ CRO platform acquisitions: Players like Charles River, Labcorp, Evotec, and Thermo Fisher expanded AI screening, in-vivo modeling, and bioanalytics capabilities.
Regulatory Progress
Drug discovery services operate across a globally distributed ecosystem.
FDA approvals in 2025: The U.S. FDA cleared 46 new CDER therapies and 8 key CBER products, including first-in-class gene therapies, sustaining an average of 48 novel approvals annually over the past five years.
Evolving regulatory landscape: Global regulators are increasingly embracing AI-driven data, real-world evidence, and reduced animal-testing approaches, speeding reviews for discovery-originated assets.
Government Funding and Policy Support
Public funding and incentives play a crucial role in sustaining early-stage innovation.
India: The government introduced a ₹5,000 crore (~USD 600 million) PRIP R&D fund for pharma and MedTech (2023-2029/30), aimed at boosting innovation-led drug discovery and technology advancement.
Japan: Government-backed initiatives through AMED and METI are funding advanced drug discovery, regenerative medicine, and AI-driven R&D, with incentives to translate academic research into commercial therapeutics.
U.S. & EU: Agencies such as NIH, BARDA, and the EU's Horizon Europe program continue backing high-risk discovery efforts, particularly in antibiotics, rare diseases, and pandemic preparedness.
Policy incentives: Tax credits and accelerated regulatory pathways-including FDA fast-track pilots and orphan-drug incentives-are reducing the overall cost and timelines from discovery to approval.
Strategic Value for R&D Organizations
Drug dTarget identification: Genomics, AI models, and disease-biology insights are used to identify promising biological targets or pathways.
Hit discovery: High-throughput and virtual screening techniques uncover initial compounds with therapeutic potential.
Lead optimization: Medicinal chemistry and lab testing refine hits to enhance efficacy, safety, and ADMET profiles.
Preclinical development: Toxicology, pharmacokinetics, and formulation studies are conducted, often through specialized CRO partners.
IND/CTA & clinical transition: Regulatory submissions enable progression into clinical trials, led by in-house teams or strategic partners.
Get Customization in the report as per your requirements:- https://www.datamintelligence.com/customize/drug-discovery-services-market?kb
Drug Discovery Research Process
✅ The modern drug discovery pathway typically follows these stages:
Target identification: Genomics, AI models, and disease-biology insights are used to identify promising biological targets or pathways.
✅ Hit discovery: High-throughput and virtual screening techniques uncover initial compounds with therapeutic potential.
✅ Lead optimization: Medicinal chemistry and lab testing refine hits to enhance efficacy, safety, and ADMET profiles.
✅ Preclinical development: Toxicology, pharmacokinetics, and formulation studies are conducted, often through specialized CRO partners.
✅ IND/CTA & clinical transition: Regulatory submissions enable progression into clinical trials, led by in-house teams or strategic partners.
Product Launches and Partnerships
Multiple AI-discovered and AI-assisted drug candidates advanced into clinical trials during 2024-2025, particularly in oncology and fibrosis.
Novel antibody-drug conjugates (ADCs), cell therapies, and gene therapies launched in 2025, many emerging from discovery-services-supported pipelines.
Technology partnerships
✦ Merck KGaA & Siemens: Partnered to connect AI-driven drug discovery with digital-twin and smart manufacturing platforms, enabling end-to-end development workflows.
✦ Pharma-AI collaborations: Alliances such as Roche-Recursion and AstraZeneca-BenevolentAI are accelerating target identification and compound discovery by combining proprietary datasets with AI platforms.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription?kb
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
Contact Us For Custom Research: https://www.datamintelligence.com/custom-research?kb
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Japan Drug Discovery Services Market Outlook (2026-2033): Key Developments and Investment Opportunities, Investment here
News-ID: 4380548 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP

Bioprocess Automation and Control Software Market Set for Explosive Growth to US …
The Bioprocess Automation and Control Software Market reached USD 4.96 billion in 2024 and is expected to reach USD 13.59 billion by 2032, growing at a CAGR of 13.7% during the forecast period 2025-2032.
Market growth is driven by surging demand for biologics and cell/gene therapies, rising adoption of Industry 4.0 in biomanufacturing, and the need for efficient, scalable production processes. Advancements in AI-driven process control, expanding single-use bioreactor systems, growing…
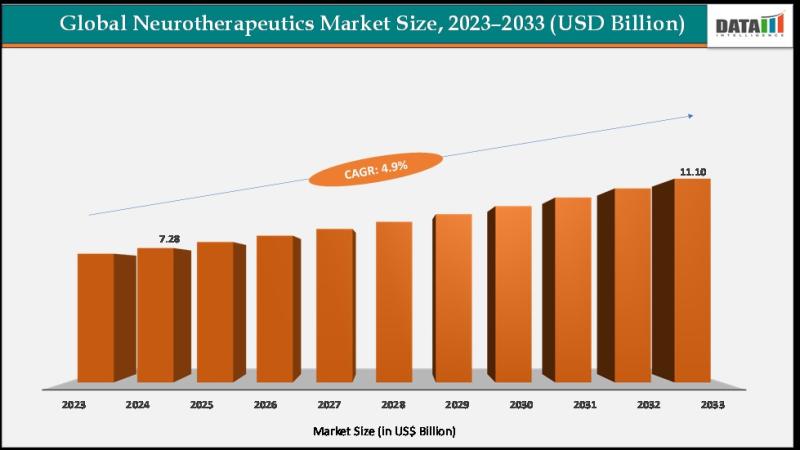
Neurotherapeutics Market to Reach US$ 11.10 Billion by 2033 at 4.9% CAGR | North …
Neurotherapeutics Market reached US$ 6.97 billion in 2023, increased to US$ 7.28 billion in 2024, and is expected to reach US$ 11.10 billion by 2033, growing at a CAGR of 4.9% during the forecast period 2025 to 2033.
The market is expanding steadily, driven by the rising global burden of neurological disorders and the rapid growth of the aging population, which significantly increases the incidence of conditions such as Alzheimer's disease,…
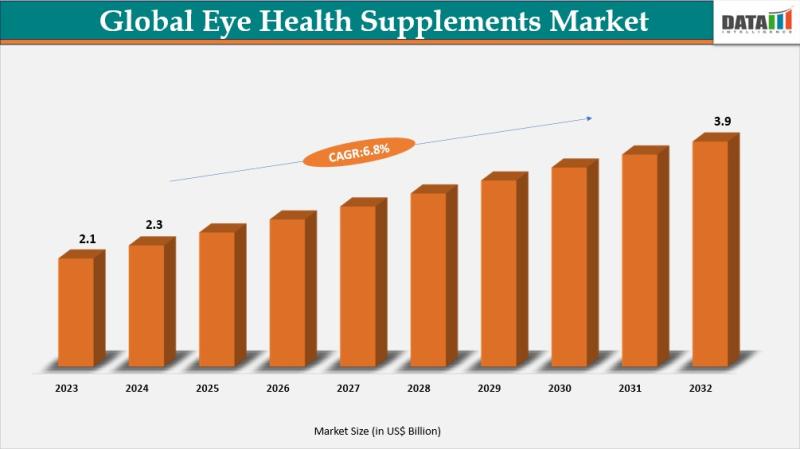
Eye Health Supplements Market to Reach US$ 3.9 Billion by 2032 at 6.8% CAGR | No …
Eye Health Supplements Market reached US$ 2.3 billion in 2024 and is expected to reach US$ 3.9 billion by 2032, growing at a CAGR of 6.8% during the forecast period 2025 to 2032.
The global eye health supplements market is witnessing steady expansion driven by rising awareness of vision care, increasing prevalence of eye-related disorders, and growing consumer focus on preventive healthcare. Demographic shifts such as the rapidly aging global population,…
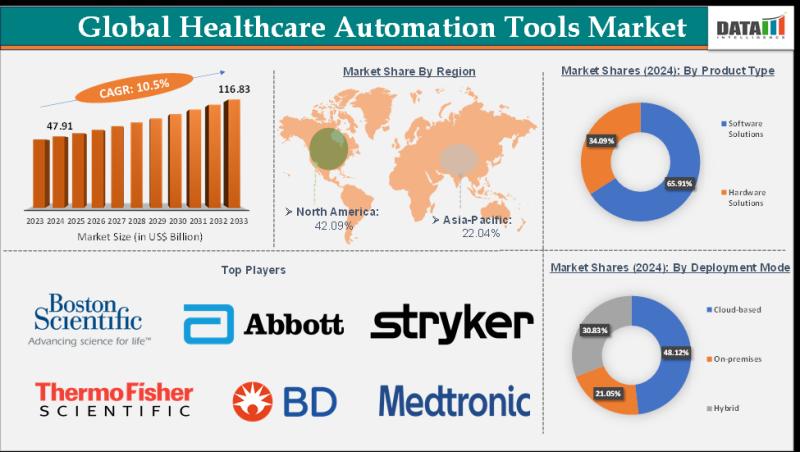
Healthcare Automation Tools Market Set for Explosive Growth to USD 116.83 Billio …
The Healthcare Automation Tools Market reached USD 47.91 billion in 2024 and is expected to reach USD 116.83 billion by 2033, growing at a CAGR of 10.5% during the forecast period 2025-2033.
Market growth is driven by the rising demand for operational efficiency in healthcare facilities, increasing adoption of AI-powered tools for diagnostics and administrative tasks, and the need to reduce clinician burnout amid staffing shortages. Advancements in robotic process automation…
More Releases for Drug
Injectable Drug Delivery Market Injectable Drug Delivery Market
Leading market research firm SkyQuest Technology Group recently released a study titled ' Injectable Drug Delivery Market Global Size, Share, Growth, Industry Trends, Opportunity and Forecast 2024-2031,' This study Injectable Drug Delivery report offers a thorough analysis of the market, as well as competitor and geographical analysis and a focus on the most recent technological developments. The research study on the Injectable Drug Delivery Market extensively demonstrates existing and upcoming…
Global Advanced Drug Delivery Systems Market Size - By Product Type(Oral Drug De …
Market Overview and Report Coverage
Advanced Drug Delivery Systems (ADDS) refer to innovative technologies designed to improve the administration and efficacy of therapeutics, enhancing the way medications are delivered to targeted areas within the body. These systems aim to optimize treatment outcomes by increasing the bioavailability, reducing side effects, and facilitating controlled drug release. Employing methods such as nanoparticles, liposomes, and implantable pumps, ADDS are revolutionizing personalized medicine and expanding therapeutic…
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Pric …
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Price, and Clinical Trials Outlook 2029 Report Highlights:
* Global Antibody Drug Conjugates Market Opportunity: > 40 Billion By 2029
* Global and Regional Antibody Drug Conjugate Market Insight
* Approved Drugs Sales Insight Global and Regional, Yearly and Quarterly, 2019 -2023
* Approved Antibody Drug Conjugates - Availability, Dosage and Price Insight
* Insight On Antibody Drug Conjugates In Clinical Trials: > 550…
Alcohol Testing And Drug Testing Equipment Market 2025 Segmentation, Application …
Market Study Report, LLC, has compiled an exhaustive research study of the ‘Alcohol Testing And Drug Testing Equipment market’, detailing every single market driver and intricately analyzing the business vertical. This ‘Alcohol Testing And Drug Testing Equipment market’ study will aid in seeking out new business opportunities and fine-tuning existing marketing strategies through insights regarding SWOT analysis, market valuation, competitive spectrum, regional share, and revenue predictions.
Alcohol abuse and drug…
How much Diabetes Drug Market Impact Worldwide Medical Drug Industry?
Diabetes Drug Market From an insight perspective, the market report focuses on various levels of analyses — industry analysis, market rank analysis, and company profiles, which together comprise and discuss basic views on the competitive landscape, high-growth regions, and countries as well as their respective regulatory policies, Types ,Applications and opportunities in the market.
Diabetes is a metabolic disorder in which the body glucose level is elevated. There are two types of diabetes…
Hepatitis Drug Market Hepatitis Drug Clinical Pipeline Report 2023
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Introduction to Hepatitis Disease
1.1 Prologue
1.1.1 History of Hepatitis
1.1.2 Causes of Hepatitis Disease
1.2 Types of Viruses which are Responsible for Hepatitis Disease
2. Global Prevalence of Hepatitis Infection
3. Available Drug Classes for Hepatitis Disease Treatment
3.1 Interferon Alfa Therapy
3.2 Protease Inhibitors Therapy
3.3 Polymerase…
