Press release
Duchenne Muscular Dystrophy Therapeutics Market to Reach US$ 6.64 Billion by 2033 at 13.2% CAGR | North America Leads with 46% Share | Key Players Amgen, Novartis, Sanofi
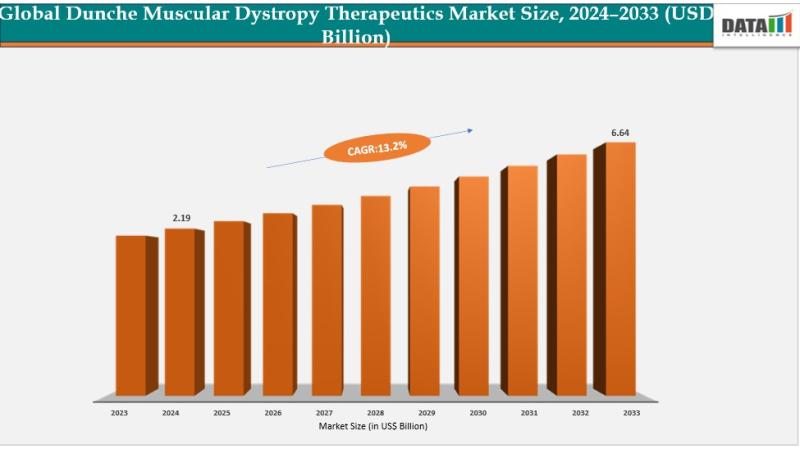
Duchenne Muscular Dystrophy Therapeutics Market reached US$ 2.19 billion in 2024 and is expected to reach US$ 6.64 billion by 2033, growing at a CAGR of 13.2% during the forecast period 2025 to 2033.The global Duchenne muscular dystrophy therapeutics market is expanding rapidly, supported by increasing research funding and strong collaborative initiatives among biotechnology companies, pharmaceutical organizations, and academic research institutions. Rising investments from both public and private sectors are accelerating the development of advanced treatment approaches, including gene editing technologies, RNA-based therapies, and regenerative cell therapies designed to address the underlying genetic cause of the disease.
Strategic partnerships such as licensing agreements, co-development programs, and technology collaborations between established pharmaceutical companies and emerging biotechnology firms are enabling knowledge sharing, reducing research and development costs, and shortening clinical development timelines. These collaborations are strengthening therapeutic innovation pipelines and improving global commercialization potential, ultimately supporting sustained long-term growth in the Duchenne muscular dystrophy therapeutics market.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/global-dunche-muscular-dystrophy-therapeutics-market?sai-v
The Duchenne Muscular Dystrophy Therapeutics Market refers to the global industry focused on the research, development, and commercialization of treatments aimed at slowing disease progression, improving muscle function, and enhancing quality of life for patients with Duchenne muscular dystrophy, including gene therapies, exon skipping therapies, corticosteroids, and supportive care approaches.
Key Developments
✅ January 2026: Across the United States and Canada, research and clinical development of Duchenne muscular dystrophy (DMD) therapeutics advanced with progress in gene therapy, exon-skipping, and novel antisense oligonucleotide (ASO) approaches designed to slow disease progression and improve muscle function.
✅ January 2026: In Europe, regulatory incentives and rare disease frameworks supported expanded clinical trials and accelerated review pathways for innovative DMD treatment candidates, increasing trial diversity and patient access opportunities.
✅ January 2026: In Japan, growing government and industry investment in rare disease research bolstered development of targeted DMD therapies, enhanced newborn screening initiatives, and strengthened patient registries to improve early diagnosis and treatment planning.
✅ December 2025: Across Asia-Pacific markets outside Japan, emerging biotechnology hubs and expanding healthcare infrastructure increased participation in global DMD clinical trials while enhancing access to investigational therapies through compassionate use programs.
✅ December 2025: Globally, integration of biomarkers, advanced delivery vectors, and CRISPR-based editing technologies improved therapeutic precision and safety profiles in next-generation DMD treatment pipelines.
✅ November 2025: In Latin America, patient advocacy and expanded specialty care services increased awareness and accessibility of DMD clinical research and emerging therapeutic options, while collaborative registries supported long-term outcome tracking.
✅ October 2025: Worldwide, combination approaches, pairing gene therapies with muscle regeneration agents, anti-inflammatory drugs, or metabolic modulators, gained traction to address complex disease mechanisms and potential treatment synergies.
Key Players
Amgen Inc. | Bristol Myers Squibb | Johnson & Johnson | Sanofi | Legend Biotech | Novartis AG | Regeneron Pharmaceuticals, Inc. | GSK plc | Others
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=global-dunche-muscular-dystrophy-therapeutics-market?sai-v
(Single User Report: USD 4350 & One Year Database Subscription: USD 12K)
Market Drivers
- Rising global prevalence of Duchenne muscular dystrophy (DMD), driven by improved diagnostic capabilities and enhanced disease awareness, is significantly increasing demand for effective therapeutic interventions.
- Growing emphasis on early genetic screening, newborn testing programs, and timely clinical intervention is accelerating uptake of targeted treatment approaches.
- Supportive regulatory incentives, orphan drug designations, and expedited approval pathways are encouraging investment in novel DMD therapeutic development.
- Advancements in gene therapy, exon-skipping technologies, and precision medicine are expanding treatment options aimed at slowing disease progression and improving patient outcomes.
- Increasing patient advocacy, real-world evidence initiatives, and expanded access programs are enhancing awareness and access to innovative therapies globally.
Industry Developments
- Development of next-generation gene therapies targeting underlying dystrophin gene mutations with improved delivery vectors and safety profiles.
- Expansion of exon-skipping and nonsense suppression therapies designed to restore dystrophin expression in specific patient populations.
- Strategic collaborations between biopharmaceutical companies, academic research institutions, and rare disease networks to accelerate clinical research and regulatory submissions.
- Increasing investment in combination therapy approaches and supportive care solutions to address muscle degeneration and long-term disease management.
- Growth of patient registries, biomarker identification, and digital monitoring tools to support personalized treatment strategies and clinical trial enrollment.
Regional Insights
North America - Holds 46% share: Driven by strong rare disease research ecosystem, high investment in gene therapy development, and early adoption of innovative DMD treatments.
Europe - Holds 28% share: Supported by established orphan drug frameworks, expanding clinical research networks, and growing access to advanced therapies.
Asia Pacific - Holds 20% share: Fueled by improving healthcare infrastructure, rising awareness of rare diseases, and increasing participation in global clinical trials.
Latin America - Holds 5% share: Growth supported by gradual improvement in rare disease care infrastructure and access to next-generation therapeutics.
Middle East & Africa - Holds 1% share: Expansion driven by growing healthcare investment, rare disease awareness initiatives, and developing specialty care services.
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/global-dunche-muscular-dystrophy-therapeutics-market?sai-v
Key Segments
By Therapeutic Type
Molecular-based therapies hold a dominant share due to their targeted mechanisms, disease-modifying potential, and growing adoption in precision treatment approaches for genetic neuromuscular disorders. Steroidal therapy represents a significant segment supported by long-standing clinical use in inflammation control, muscle strength preservation, and symptom management. NSAIDs and other supportive therapies contribute to pain management, functional improvement, and overall quality-of-life enhancement in affected patients.
By Mutation Type
Exon 51 skipping accounts for the largest share driven by broader patient eligibility and availability of approved targeted therapies. Exon 53 and exon 45 skipping represent significant segments supported by expanding research, clinical development, and regulatory progress for mutation-specific treatments. Other mutation types continue to contribute through ongoing genetic research and emerging personalized therapeutic strategies.
By Route of Administration
Intravenous administration dominates the segment due to its use in advanced biologic and gene-targeted therapies requiring controlled clinical delivery. Subcutaneous administration is gaining traction owing to improved patient convenience, reduced administration time, and development of self-injectable formulations. Other administration routes support specialized treatment protocols and emerging delivery technologies.
By Distribution Channel
Hospital pharmacies hold the largest share supported by specialist supervision, complex therapy handling, and infusion-based treatment delivery. Specialty pharmacies represent a significant and growing segment due to focused management of rare diseases, patient support programs, and distribution of high-cost targeted therapies.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Duchenne Muscular Dystrophy Therapeutics Market to Reach US$ 6.64 Billion by 2033 at 13.2% CAGR | North America Leads with 46% Share | Key Players Amgen, Novartis, Sanofi here
News-ID: 4380426 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
Next-Generation Multiple Myeloma Therapies Market to Reach US$ 37.07 Billion by …
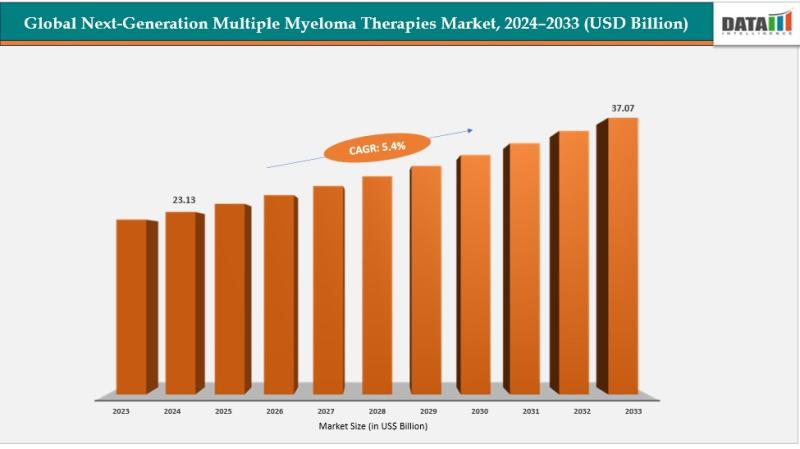
Next-Generation Multiple Myeloma Therapies Market reached US$ 22.03 billion in 2023, increased to US$ 23.13 billion in 2024, and is projected to attain US$ 37.07 billion by 2033, growing at a CAGR of 5.4% during the forecast period 2025 to 2033.
Multiple myeloma is a rare yet aggressive hematologic malignancy originating in plasma cells within the bone marrow, leading to uncontrolled cellular proliferation, bone destruction, renal impairment, and weakened immune defense.…
Disease-Modifying Therapies Market to Reach US$ 112.64 Billion by 2033 at 6.2% C …
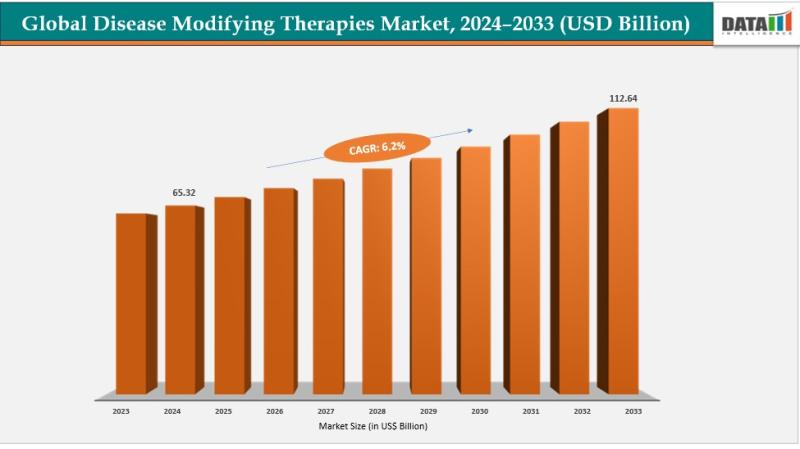
Disease-Modifying Therapies Market was valued at US$ 65.32 billion in 2024 and is projected to reach US$ 112.64 billion by 2033, expanding at a CAGR of 6.2% during the forecast period 2025 to 2033.
The market is witnessing sustained growth driven by rapid advancements in biologics, small-molecule therapeutics, and precision medicine approaches that aim to slow or halt disease progression rather than only manage symptoms. Innovative biologic therapies, including monoclonal antibodies…
Industrial Automation Market is Except to reach USD 372.70 billion by 2032 With …
The global Industrial automation market size was valued at USD 196.94 billion in 2024 and is expected to reach USD 372.70 billion by 2032, at a CAGR of 8.30% during the forecast period 2024-2032
Industrial automation market growth is driven by smart factories, labor shortages, rising energy costs, AI integration, demand for precision manufacturing, and government-backed digitalization initiatives improving productivity, safety, and operational efficiency.
Get a Free Sample PDF Of This Report…
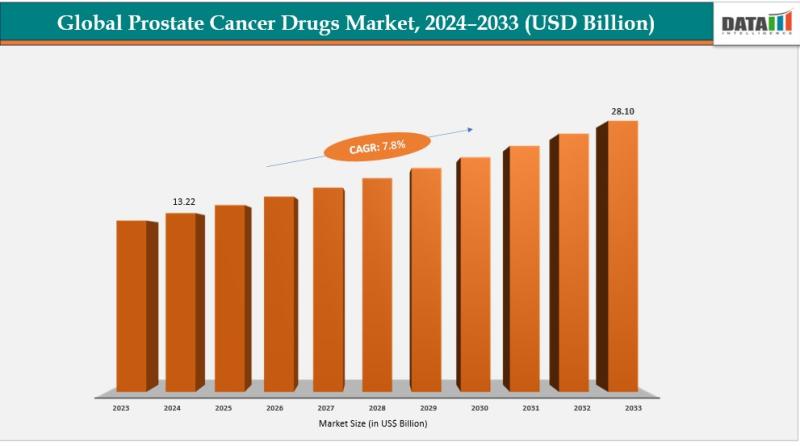
Prostate Cancer Drugs Market to Reach US$ 28.10 Billion by 2033 at 8.1% CAGR | N …
Prostate Cancer Drugs Market was valued at US$ 13.22 billion in 2024 and is expected to reach US$ 28.10 billion by 2033, growing at a CAGR of 8.1% during the forecast period 2025 to 2033.
The market is expanding steadily due to the rising global incidence of prostate cancer, primarily driven by the rapid growth of the aging male population. Prostate cancer risk increases significantly after the age of 50 and…
More Releases for Duchenne
Duchenne Muscular Dystrophy (DMD) Market Growth in 2034
Market Overview
The Duchenne Muscular Dystrophy (DMD) Market is expanding rapidly as advances in genetic medicine, exon-skipping therapies, gene therapy platforms, and improved diagnostic capabilities reshape treatment options for this severe, progressive neuromuscular disorder.
DMD is caused by mutations in the dystrophin gene, leading to muscle degeneration beginning in early childhood. Growing awareness among clinicians and caregivers, widespread adoption of next-generation sequencing (NGS), and increasing availability of disease-modifying therapies have significantly strengthened…
Duchenne Muscular Dystrophy: Core Growth Enabler in the Rising Prevalence Of Chr …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
What Will the Duchenne Muscular Dystrophy Industry Market Size Be by 2025?
The market size for Duchenne Muscular Dystrophy has shown significant expansion recently, burgeoning from $1.16 billion in 2024 to $1.25 billion in 2025 at a compound annual growth rate (CAGR) of 7.9%. The impressive growth in the…
Shaping the Duchenne Muscular Dystrophy (DMD) Therapeutics Market in 2025: Bit B …
How Big Is the Duchenne Muscular Dystrophy (DMD) Therapeutics Market Expected to Be, and What Will Its Growth Rate Be?
The Duchenne muscular dystrophy (DMD) therapeutics market will grow from $11.95 billion in 2024 to $16.45 billion in 2025, at a CAGR of 37.6%. The growth is attributed to the increasing prevalence of Duchenne muscular dystrophy, rising awareness of treatment options, healthcare spending, and government initiatives.
The Duchenne muscular dystrophy (DMD) therapeutics…
Duchenne Muscular Dystrophy Pipeline Outlook Report 2024
DelveInsight's, "Duchenne Muscular Dystrophy Pipeline Insight 2024" report provides comprehensive insights about 75+ companies and 75+ pipeline drugs in the Duchenne Muscular Dystrophy pipeline landscape. It covers the Duchenne Muscular Dystrophy pipeline drug profiles, including Duchenne Muscular Dystrophy clinical trials and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
…
Duchenne Muscular Dystrophy Treatmcent Market
Global Duchenne Muscular Dystrophy Treatmcent Market Set for Robust Growth During Forecast Period
The global Duchenne Muscular Dystrophy Treatment Market is poised to witness significant growth at a high Compound Annual Growth Rate (CAGR) during the forecast period of 2023 to 2030. Duchenne muscular dystrophy (DMD) stands as a genetic disorder marked by progressive muscle degeneration and weakness, with therapeutics aimed at addressing the absence of dystrophin, a crucial protein in…
Duchenne Muscular Dystrophy (DMD) Drugs Market Report
As per the research conducted by GME, the Duchenne Muscular Dystrophy (DMD) Drugs Market will grow with a CAGR value of 41.3 percent by 2026. The global market for Duchenne muscular dystrophy is foreseen to grow due to accelerated research and development activities, expanded patient awareness for effective treatments, and the launch of new medications Moreover, the disease's increasing prominence is likely to drive the global duchenne muscular dystrophy market…