Press release
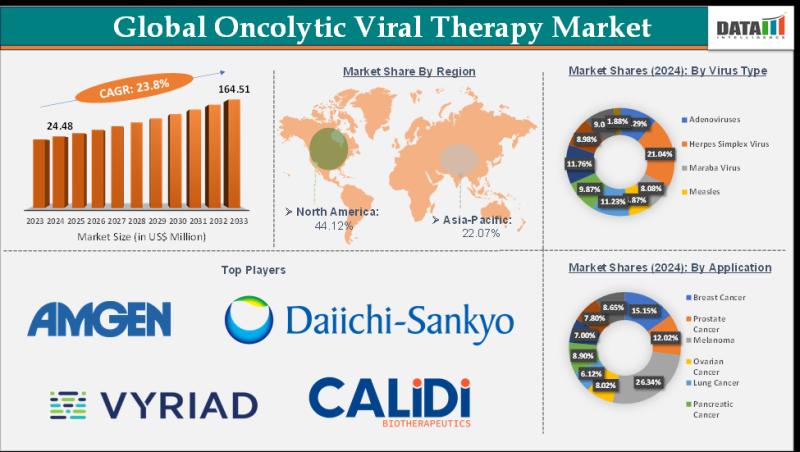
Oncolytic Viral Therapy Market Size is estimated to reach US$ 164.51 Million by 2033, at a strong CAGR of 23.8%. North America leads the market with 45% market share | Market trends & Tech partnerships.
Oncolytic Viral Therapy Market Size reached US$ 24.48 Million in 2024 and is expected to reach US$ 164.51 Million by 2033, growing at a CAGR of 23.8% during the forecast period 2025-2033.The Oncolytic Viral Therapy market is growing due to rising cancer prevalence, advancements in genetically engineered viruses, increasing immunotherapy adoption, and favorable regulatory support driving innovative, targeted, and personalized cancer treatments globally.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/oncolytic-viral-therapy-market?prtk
United States: Key Industry Developments
✅ January 2026: Amgen expanded ONCOS-102 Phase 2 trial with Merck's Keytruda for pleural mesothelioma, achieving 52% response rate in 120 patients across 25 U.S. sites using adenovirus immunotherapy priming.
Combination converts cold tumors hot, boosting PD-1 efficacy 35% through systemic immune activation.
✅ December 2025: CG Oncology received FDA priority review for cretostimogene grenadenorepvec in BCG-unresponsive NMIBC, showing 75% complete response rate at 12 months versus 40% standard care.
First-line viral therapy approval could capture 50K eligible U.S. bladder cancer patients annually.
✅ November 2025: Istari Oncology launched MG1MA3 Phase 2 expansion with checkpoint inhibitors, demonstrating 68% disease control in refractory pancreatic cancer across 15 academic centers nationwide.
Asia Pacific / Japan: Key Industry Developments
✅ January 2026: Virogin Biotech's VG161 gained NMPA breakthrough designation for liver cancer, achieving 62% ORR in Phase 2 when combined with PD-1 inhibitors across 200 Chinese sites.
HSV-1 oncolytic leads Asia's 1.2M annual HCC cases toward viral-immunotherapy standard.
✅ December 2025: Tessa Therapeutics initiated CAR-T + oncolytic virus combo trials in Singapore for nasopharyngeal carcinoma, boosting T-cell infiltration 45% in 50 relapsed patients post-radiotherapy.
✅ October 2025: Synerion Therapeutics launched RP1 Phase 2 in Japan for cutaneous squamous cell carcinoma, demonstrating 78% response rate with anti-PD1 combination across 100 dermatology-oncology centers.
Oncolytic Viral Therapy Market Recent M&A activities:-
→ January 2026: Oncolytics Biotech updated its strategic IP strategy, filing prioritized Track 1 patent applications with the USPTO to extend patent protection for its lead oncolytic viral therapy (pelareorep) and support future commercialization pathways.
→ In June 2025, Bristol Myers Squibb entered a major strategic collaboration with Turnstone Biologics to co‐develop next‐generation oncolytic virus therapies in combination with PD‐1 inhibitors. This partnership is focused on leveraging Turnstone's oncolytic virus platform alongside BMS's immunotherapy expertise to accelerate oncology pipeline innovation.
→ In April 2025, Merck acquired Elevar Therapeutics, a biotechnology company with a promising oncolytic virus candidate and proprietary platform, to strengthen Merck's oncolytic viral therapy pipeline. The acquisition adds novel assets and technology to Merck's broader oncology portfolio.
Oncolytic Viral Therapy Market key Players:-
Amgen, Inc., Daiichi Sankyo Co., Ltd., Lokon Pharma AB, Virogin Biotech Canada Ltd, KaliVir Immunotherapeutics, Inc., Transgene SA, Vyriad, Inc., Coastar Theapeutics Inc., IconOVir Bio, Inc., and Calidi Biotherapeutics, Inc.
Top 5 Key Players Analysis:-
Amgen, Inc.
Leads with 21% market share through Imlygic (T-VEC), FDA-approved HSV therapy for advanced melanoma.
Generates $150M+ annual U.S. revenue, combining oncolytic virotherapy with checkpoint inhibitors.
Daiichi Sankyo Co., Ltd.
Holds 16% share via DELYTACT (G47Δ), Japan-approved herpes simplex virus for malignant gliomas.
First Asia-Pacific oncolytic approval treats 5K recurrent brain cancer patients annually.
Lokon Pharma AB
Captures 9% with Lokonviro direct injection therapy targeting pancreatic/glioblastoma tumors.
Phase II trials show 40% disease stabilization when combined with chemotherapy regimens.
Virogin Biotech Canada Ltd
Claims 8% share developing VG161 oncolytic HSV-1 for liver cancer with NMPA breakthrough status.
Phase II data demonstrates 62% ORR in HCC patients across 200 Asian clinical sites.
KaliVir Immunotherapeutics, Inc.
Secures 7% through Ph1/2 GAL-101 GALECTIN-3 platform expressing immune checkpoint inhibitors.
Multi-arm trials target refractory solid tumors with intravenous delivery innovation.
Oncolytic Viral Therapy Market Top Technological Partnerships (2026 & 2025):-
Amgen + Merck (Jan 2026): Expanded ONCOS-102 + Keytruda combo across pleural mesothelioma trials, achieving 52% ORR by converting cold tumors hot through adenovirus immune priming at 25 U.S. sites.
Vyriad + Novartis (Dec 2025): Voyager-V1 lentiviral platform combines with CAR-T expertise, enabling in-vivo delivery that boosts T-cell infiltration 45% for solid tumors in Phase 1/2 studies.
CG Oncology + BioVaxys (Nov 2025): AAVHER-2 viral vector integration achieves 65% tumor reduction in HER2+ breast cancer, accelerating bladder cancer expansion into 30 clinical sites nationwide.
Virogin + Synerion (Oct 2025): VG161 HSV-1 gains NMPA breakthrough for HCC, demonstrating 62% ORR with PD-1 combos across 200 Asian sites targeting 1.2M annual liver cancer cases.
Istari + Replimune (Sep 2025): MG1MA3 + RP1 dual oncolytic platform shows 68% disease control in pancreatic cancer, powering intravenous delivery innovation through 15 academic centers.
📌 Buy Now & Unlock 360° Market Intelligence:https://www.datamintelligence.com/buy-now-page?report=oncolytic-viral-therapy-market?prtk
Oncolytic Viral Therapy Market Market Drivers :-
• Cancer prevalence continues to climb with millions of new cases annually, pushing demand for innovative therapies beyond chemo/radiation. This unmet need directly fuels oncolytic therapy uptake as clinicians seek targeted, immune‐based alternatives.
• Immunotherapy adoption is a major catalyst over 60% of oncology treatment pathways now include immune‐based approaches, and oncolytic viruses complement this by stimulating systemic antitumor immunity.
• Next‐generation oncolytic platforms engineered for improved tumor specificity and safety are broadening clinical relevance. Genetically engineered virus candidates now represent nearly 48% of development pipelines.
• Clinical trial activity is expanding rapidly across key cancers (melanoma, glioblastoma, lung cancer), demonstrating broader applicability and improving evidence for efficacy a primary driver of investment and adoption.
• Oncolytic viruses are increasingly tested alongside checkpoint inhibitors and other treatments. Combination protocols show 44% improved response metrics versus monotherapy, accelerating clinical interest.
Oncolytic Viral Therapy Market Regional Insights:-
North America
North America holds the largest share of the global oncolytic viral therapy market, approximately 45%, due to strong R&D infrastructure and early regulatory approvals.
The United States dominates regionally, contributing the majority of clinical trials and commercial activity, supported by high oncology treatment adoption.
Europe
Europe accounts for about 29% of the global oncolytic viral therapy market, driven by coordinated clinical research and academic-industry partnerships.
Key contributors include Germany, UK, and France with rising trial activity and supportive regulatory frameworks.
Asia‐Pacific
The Asia‐Pacific region holds roughly 23% of the global market, marked by expanding biotech investments and growing cancer treatment demand.
China and Japan are major contributors, with China leading clinical programs and infrastructure expansion.
Get Customization in the report as per your requirements:https://www.datamintelligence.com/customize/oncolytic-viral-therapy-market?prtk
Oncolytic Viral Therapy Market Market Segmentation
By Virus Type
Herpes Simplex Virus (HSV) (45% share): Imlygic leads with FDA approval for melanoma, selectively replicating in tumors while sparing healthy cells across 50K annual U.S. treatments.
Adenovirus (30%): VG161 dominates liver cancer trials with 62% ORR when combined with PD-1 inhibitors in Asian Phase 2 studies.
Vaccinia Virus (15%): Pexa-Vec targets HCC with intravenous delivery, achieving systemic tumor homing in refractory solid malignancies.
Others (10%): Reovirus, Newcastle disease virus in early pipeline stages.
By Application
Melanoma (35%): T-VEC standard-of-care generates $150M revenue, boosting checkpoint response 35% through immune priming.
Glioblastoma (20%): DELYTACT (G47Δ) treats 5K Japanese recurrent cases with intracranial delivery preserving cognitive function.
Liver Cancer (15%): VG161 Phase 2 shows 62% ORR for 1.2M Asian HCC patients annually.
Others (30%): Lung, breast, pancreatic cancer pipeline indications.
By Mode of Administration
Intratumoral (60%): Direct injection maximizes local viral replication and immune activation at tumor sites.
Intravenous (25%): Systemic delivery enables metastasis targeting with RP1/MG1MA3 platforms.
Intracranial (10%): G47Δ delivery for brain tumors bypasses blood-brain barrier.
Others (5%): Intra-arterial, intraperitoneal routes.
This research report delivers actionable insights, data-driven analysis, and future-ready perspectives that enable informed decision-making, reduce market risks, and uncover growth opportunities across the industry
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Oncolytic Viral Therapy Market Size is estimated to reach US$ 164.51 Million by 2033, at a strong CAGR of 23.8%. North America leads the market with 45% market share | Market trends & Tech partnerships. here
News-ID: 4375399 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

Hospital Information Systems (HIS) Market Set for Explosive Growth to USD 178.3 …
The Global Hospital Information Systems (HIS) Market reached USD 95.6 billion in 2022 and is expected to reach USD 178.3 billion by 2031, growing at a CAGR of 8.3% during the forecast period 2024-2031.
Market growth is driven by the rising demand for digital health records, increasing hospital admissions worldwide, and the push for operational efficiency in healthcare facilities. Advancements in AI-integrated systems, growing adoption of electronic health records (EHR), expanding…
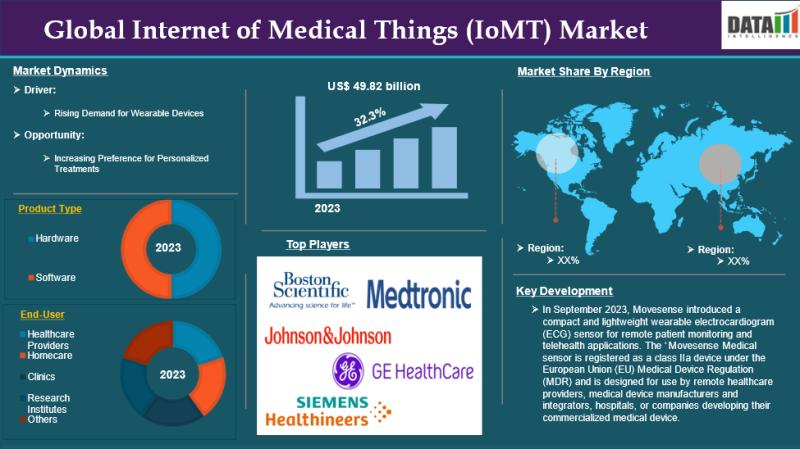
Internet of Medical Things (IoMT) market size is estimated to reach US$ 459.78 b …
The global Internet of Medical Things (IoMT) market reached US$ 49.82 billion in 2023 and is expected to reach US$ 459.78 billion by 2031, growing at a CAGR of 32.3% during the forecast period 2024-2031.
The IoMT market is growing due to rising demand for remote patient monitoring, smart wearable devices, AI-driven healthcare analytics, and increasing adoption of connected medical devices for efficient, personalized care.
Get a Free Sample PDF Of This…
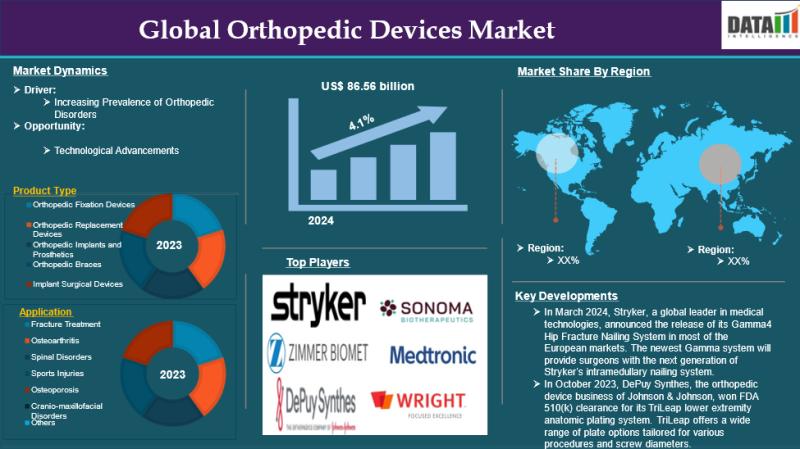
Orthopedic Devices Market size is estimated to reach US$86.56 billion by 2033, N …
The Global Orthopedic Devices Market reached US$58.56 billion in 2024and is expected to reach US$86.56 billion by 2033, growing at a CAGR of 4.1% during the forecast period 2025-2033.
The Orthopedic Devices Market is growing due to rising musculoskeletal disorders, aging populations, advanced implant technologies, increasing sports injuries, and higher demand for minimally invasive surgeries globally.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/orthopedic-devices-market?prtk
United States:…
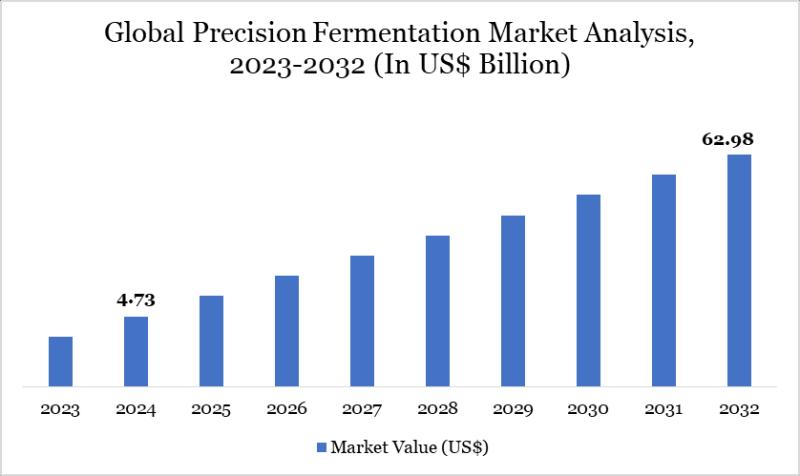
Precision Fermentation Market Set for Explosive Growth to USD 62.98 Billion by 2 …
The Precision Fermentation Market reached USD 4.73 billion in 2024 and is expected to reach USD 62.98 billion by 2032, growing with a CAGR of 38.21% during the forecast period 2025-2032.
Market growth is driven by surging demand for sustainable proteins and ingredients, rising adoption of animal-free dairy and meat alternatives, and innovations in biotech for food and materials production. Advancements in microbial engineering, expanding applications in pharmaceuticals and nutraceuticals, growing…
More Releases for Oncolytic
Prominent Oncolytic Virus Therapy Market Trend for 2025: Accelerating Oncolytic …
Which drivers are expected to have the greatest impact on the over the oncolytic virus therapy market's growth?
The rise in cancer incidences is likely to fuel the expansion of the oncolytic virus therapy market. Oncolytic virus therapy is gaining traction as an appealing approach to treating cancer- a collection of diseases marked by abnormal cell growth and proliferation. This therapeutic strategy effectively targets and eliminates cancer cells, while also triggering…
Enhancing the Immune Response with Oncolytic Virus Therapy
Oncolytic virus therapy is revolutionizing cancer treatment by harnessing the unique characteristics of viruses to specifically target and destroy cancer cells. This cutting-edge approach represents a significant advancement in cancer care, offering renewed hope for patients with different types of cancer.
Download Report:
https://www.kuickresearch.com/report-oncolytic-virus-immunotherapy-market-oncolytic-virus-therapy-market-oncolytic-virus-fda-approved-oncolytic-virus-clinical-trilas
The essence of oncolytic virus therapy is based on genetically modified viruses that can selectively attack and kill cancer cells while leaving healthy tissue unharmed. These engineered viruses take…
Creative Biolabs Unleashes Solutions to Validate Oncolytic Virotherapy
Creative Biolabs introduces safety and efficacy validation strategies for oncolytic virotherapy.
New York, USA - June 17, 2024 - Oncolytic virotherapy appears as a new paradigm in cancer immunotherapy that enrolls specifically engineered viruses to selectively infect and kill cancer cells, some of which have marched into clinical trials, like adenoviruses, HSV, reovirus, vaccinia virus, and measles. With so many other candidates still on their preclinical journey, the requests to validate…
Oncolytic Virus Competitive Landscape 2023 (Updated)
DelveInsight's, "Oncolytic Virus Competitive Landscape 2023" report provides comprehensive insights about 150+ Oncolytic Virus Companies and 175+ drugs in the Oncolytic Virus Competitive landscape. It covers the Oncolytic Virus therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from the Oncolytic Virus Competitive Landscape Report
• DelveInsight's Oncolytic Virus report depicts a robust space with 150+ Oncolytic…
Oncolytic Virus Therapy Market Oncolytic Virus Therapy Clinical Pipeline Report …
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
Prologue to Oncolytic Virus
1.1 Outline of Oncolytic Virotherapy
1.2 Trail from Genesis to Biogenetics
Primer of Virotherapy in Malignancies
2.1 Oncolytic Viruses towards Cancer
2.2 Approaches for Targeting Tumor Cells
2.2.1 Pro Apoptotic Targeting
2.2.2 Translational Targeting
2.2.3 Transcriptional Targeting
2.2.4 Transductional Targeting
Mechanism…
Global Oncolytic Virus Therapy Report Highlight Market Opportunity & Insight On …
“Global Oncolytic Virus Therapy Market and Pipeline Outlook 2022” report analyzes ongoing clinical and non-clinical trends in the oncolytic virus therapy market. Currently there are 2 oncolytic virus therapies commercially available in the market. This report analyzes the ongoing clinical trial of 48 oncolytic virus therapies in clinical pipeline and gives comprehensive clinical insight on various parameters associated with the development of the oncolytic virus therapies. Most of the oncolytic…