Press release
Cefadroxil Manufacturing Plant Setup Cost 2026: Income, Expenses & Profitability
The global pharmaceutical industry is experiencing sustained growth driven by rising prevalence of bacterial infections, increasing antibiotic consumption in emerging economies, expansion of generic pharmaceutical production, and growing access to healthcare services worldwide. At the core of these developments lies a critical antibiotic active pharmaceutical ingredient-cefadroxil. As healthcare systems expand and generic antibiotic markets mature across developing regions, establishing a cefadroxil manufacturing plant presents a strategically compelling business opportunity for pharmaceutical entrepreneurs and API producers seeking to capitalize on consistent antibiotic demand and established therapeutic applications driving stable market growth.IMARC Group's report, "Cefadroxil Manufacturing Plant Project Report 2026: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue," offers a comprehensive guide for establishing a manufacturing plant. The cefadroxil manufacturing plant cost report offers insights into the manufacturing process, financials, capital investment, expenses, ROI, and more for informed business decisions.
Request for a Sample Report: https://www.imarcgroup.com/cefadroxil-manufacturing-plant-project-report/requestsample
Market Overview and Growth Potential
The global cefadroxil market demonstrates steady growth trajectory, valued at USD 350.48 Million in 2025. According to IMARC Group's comprehensive market analysis, the market is projected to reach USD 486.04 Million by 2034, exhibiting a consistent CAGR of 3.7% from 2026-2034. This sustained expansion is driven by rising prevalence of bacterial infections, increasing antibiotic consumption in emerging economies, expansion of generic pharmaceutical production, and growing access to healthcare services worldwide.
Cefadroxil is a first-generation cephalosporin antibiotic extensively used for treating bacterial infections caused by susceptible gram-positive and gram-negative organisms. The drug eliminates bacteria through inhibition of bacterial cell wall synthesis. Cefadroxil constitutes the first-line treatment for infections of the respiratory tract, urinary tract, skin, soft tissues, and bones. Due to its excellent pharmacokinetic profile, long half-life, and oral bioavailability, cefadroxil is administered in capsule, tablet, and suspension forms. The drug is valued for its safety profile, therapeutic efficacy, and broad applicability across adult and pediatric patient populations. Cefadroxil remains an important active pharmaceutical ingredient in the global generic antibiotics market, with industrial-scale production supporting international pharmaceutical supply chains.
Market growth is fueled by steady demand for antibiotics and improved healthcare access in developing nations. In 2025, 468 hospitals joined India's Central Government Health Scheme (CGHS), significantly broadening access for government beneficiaries. This expansion in healthcare infrastructure increased demand for essential antibiotics like cefadroxil, as more patients gained access to reliable treatments for bacterial infections across newly empanelled hospitals. Rising awareness among patients and healthcare providers regarding proper bacterial infection management, combined with improved medicine accessibility, supports steady cephalosporin demand. Hospital procurement programs and public health initiatives contribute to market stability.
Plant Capacity and Production Scale
The proposed cefadroxil manufacturing facility is designed with an annual production capacity ranging between 50-200 MT per year, enabling economies of scale while maintaining operational flexibility. This capacity range allows manufacturers to serve diverse market segments-from pharmaceutical formulation companies and generic drug manufacturers to hospital supply chains and contract manufacturing organizations-ensuring steady demand and consistent revenue streams across multiple distribution channels serving domestic and international antibiotic markets.
Financial Viability and Profitability Analysis
The cefadroxil manufacturing business demonstrates healthy profitability potential under normal operating conditions. The financial projections reveal margins supported by stable antibiotic demand and established therapeutic positioning:
• Gross Profit Margins: 15-25%
• Net Profit Margins: 5-10%
These margins reflect the mature nature of cephalosporin antibiotics while demonstrating viable profitability supported by consistent global antibiotic consumption, essential medicine status in healthcare systems, established generic manufacturing base, and predictable demand patterns across both developed and emerging pharmaceutical markets. The project demonstrates reasonable return on investment (ROI) potential, making it an attractive proposition for pharmaceutical companies seeking to establish or expand API production capabilities in the growing generic antibiotics sector.
Operating Cost Structure
Understanding the operating expenditure (OpEx) is crucial for effective financial planning and cost management. The cost structure for a cefadroxil manufacturing plant reflects pharmaceutical API production economics:
• Raw Materials: 45-55% of total OpEx
• Utilities: 15-20% of OpEx
• Other Expenses: Including labor, packaging, transportation, quality control, regulatory compliance, maintenance, depreciation, and taxes
7-ACA (7-aminocephalosporanic acid) constitutes the primary raw material, representing the dominant cost component. Additional inputs include solvents, reagents, and catalysts essential for chemical synthesis processes. The utilities component reflects energy requirements for chemical reactions, crystallization, filtration, and drying operations. The cost structure emphasizes the critical importance of securing reliable 7-ACA supply through established chemical intermediates suppliers, optimizing reaction yields, and maintaining stringent quality control throughout manufacturing to ensure pharmaceutical-grade API production meeting international pharmacopoeial standards.
Buy Now: https://www.imarcgroup.com/checkout?id=13819&method=2175
Capital Investment Requirements
Setting up a cefadroxil manufacturing plant requires substantial capital investment across several critical categories:
Land and Site Development: Selection of an optimal location meeting pharmaceutical manufacturing standards, with access to chemical intermediates suppliers, proximity to pharmaceutical formulation markets, and robust infrastructure including reliable utilities, waste management systems, and regulatory compliance frameworks. The site must accommodate GMP (Good Manufacturing Practices) requirements and environmental regulations governing pharmaceutical chemical production.
Machinery and Equipment: The largest portion of capital expenditure (CapEx) covers specialized pharmaceutical processing equipment essential for API production. Key machinery includes:
• Reactors for chemical synthesis
• Centrifuges for solid-liquid separation
• Filters for product purification
• Dryers for moisture removal
• Milling systems for particle size control
• Analytical instruments for quality testing
Civil Works: Building construction, cleanroom facilities, factory layout optimization meeting GMP standards, and infrastructure development designed for pharmaceutical operations, ensuring product quality, worker safety, and regulatory compliance throughout production processes.
Other Capital Costs: Pre-operative expenses, machinery installation and validation, pharmaceutical certifications, quality control laboratory establishment, initial working capital requirements, and contingency provisions for regulatory compliance and unforeseen circumstances during plant establishment.
Major Applications and Market Segments
Cefadroxil API finds extensive applications across diverse pharmaceutical sectors and healthcare applications:
Pharmaceutical Formulations: Cefadroxil API supports production of oral antibiotics delivering consistent potency, stability, and bioavailability in finished dosage forms including capsules, tablets, and dry syrups for pediatric and adult populations.
Hospital and Clinical Use: The antibacterial compound serves as a dependable treatment agent for community-acquired infections within standardized therapeutic protocols, offering reliable efficacy against susceptible bacterial pathogens.
Generic Drug Manufacturing: Cefadroxil enables production of affordable antibiotics for generic pharmaceutical markets in both regulated and semi-regulated regions, supporting access to essential medicines in cost-sensitive markets.
Export-Oriented Supply Chains: The API serves international pharmaceutical supply chains meeting pharmacopoeial standards, enabling export opportunities across multiple regulatory jurisdictions and geographic markets.
End-use sectors encompass pharmaceutical formulation manufacturers, hospital supply chains, generic drug companies, and international pharmaceutical markets, all contributing to sustained API demand.
Why Invest in Cefadroxil Manufacturing?
Several compelling factors make cefadroxil manufacturing an attractive investment opportunity:
Sustained Demand for Antibiotics: Cefadroxil represents a widely prescribed antibiotic with established clinical effectiveness and proven therapeutic applications. As a first-generation cephalosporin, it maintains consistent demand driven by ongoing bacterial infection treatment needs across global healthcare systems.
Strong Generic Market Presence: The molecule benefits from multiple generic manufacturers and well-established production pathways, ensuring long-term market relevance and predictable demand patterns across both mature and emerging pharmaceutical markets.
Scalable API Production: Manufacturing processes for cefadroxil permit scalable batch operations with well-controlled quality parameters. Established synthesis routes and process optimization opportunities enable efficient production scaling to meet market demand.
Regulatory Acceptance: The compound maintains acceptance across multiple pharmacopoeias, facilitating market access in various regulatory jurisdictions. Well-defined quality standards and testing procedures support regulatory compliance across international markets.
Stable Pricing Structure: Long-term market presence enables predictable pricing dynamics and volume-based production planning. Mature generic markets provide revenue stability and established customer relationships supporting business sustainability.
Ask Analyst for Customization: https://www.imarcgroup.com/request?type=report&id=13819&flag=C
Manufacturing Process Excellence
The cefadroxil manufacturing process involves several controlled stages ensuring pharmaceutical-grade quality and regulatory compliance:
• Chemical Synthesis: 7-ACA undergoes controlled chemical reactions with appropriate reagents to form cefadroxil
• Crystallization: Product is crystallized from solution to achieve desired purity and particle characteristics
• Filtration: Solid product is separated from mother liquor through filtration processes
• Drying: Filtered product undergoes controlled drying to achieve target moisture specifications
• Milling: Dried material is milled to required particle size distribution for formulation applications
• Quality Testing: Finished API undergoes comprehensive testing for identity, purity, potency, and impurities per pharmacopoeial standards
• Packaging: Tested and approved API is packaged in appropriate containers ensuring stability during storage and transportation
Industry Leadership
The global cefadroxil industry includes established pharmaceutical manufacturers with API production capabilities. Key industry players include:
• Union Chempharma
• NCPC
• Qilu Antibiotics
• Lupin Pharmaceuticals
• Fukang
These companies serve diverse end-use sectors including pharmaceutical formulation manufacturers, hospitals, and international markets, demonstrating the broad applicability of cefadroxil API production.
Recent Industry Developments
January 2025: A clinical study published in Open Forum Infectious Diseases evaluated cefadroxil as oral transitional therapy for staphylococcal and streptococcal bloodstream infections. Findings demonstrate that cefadroxil achieves comparable outcomes to other oral antibiotics such as linezolid and TMP/SMX in mortality, readmission, and recurrence metrics, while offering less frequent dosing benefits due to its longer half-life.
September 2024: A study published in Antimicrobial Stewardship & Healthcare Epidemiology highlighted oral cefadroxil as a potential treatment for gram-positive osteoarticular infections in adults, including prosthetic joint infections and osteomyelitis. Researchers emphasize the need for larger comparative and prospective studies to confirm efficacy and safety, underscoring cefadroxil's emerging role in managing complex bone and joint infections.
Browse Related Reports:
• Azodicarbonamide Production Plant Cost: https://industrytoday.co.uk/chemicals/azodicarbonamide-production-plant-cost-report-2025-capexopex-analysis-with-profitability-forecasts
• Veneer Production Plant Cost: https://industrytoday.co.uk/chemicals/veneer-production-plant-cost-report-2025-unit-setup-economics-and-financial-outlook
• Copper Sulphate Production Cost: https://industrytoday.co.uk/chemicals/copper-sulphate-production-cost-report-2025-feasibility-study-plant-setup-and-profitability-insights
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company excels in understanding its client's business priorities and delivering tailored solutions that drive meaningful outcomes. We provide a comprehensive suite of market entry and expansion services. Our offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape, and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No: (D) +91 120 433 0800
United States: (+1-201-971-6302)
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Cefadroxil Manufacturing Plant Setup Cost 2026: Income, Expenses & Profitability here
News-ID: 4369323 • Views: …
More Releases from IMARC Group
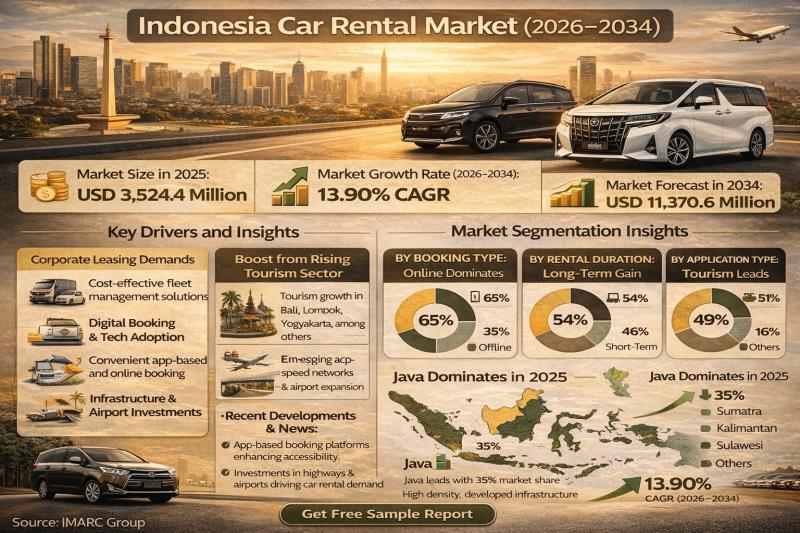
Indonesia Car Rental Market Size to Hit USD 11,370.6 Million by 2034 | Expanding …
Indonesia Car Rental Market Overview
According to IMARC Group's report titled "Indonesia Car Rental Market Size, Share, Trends and Forecast by Booking Type, Rental Duration, Application Type, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
The Indonesia car rental market size was valued at USD 3,524.4 Million in 2025. IMARC Group projects this market to reach USD 11,370.6 Million by…
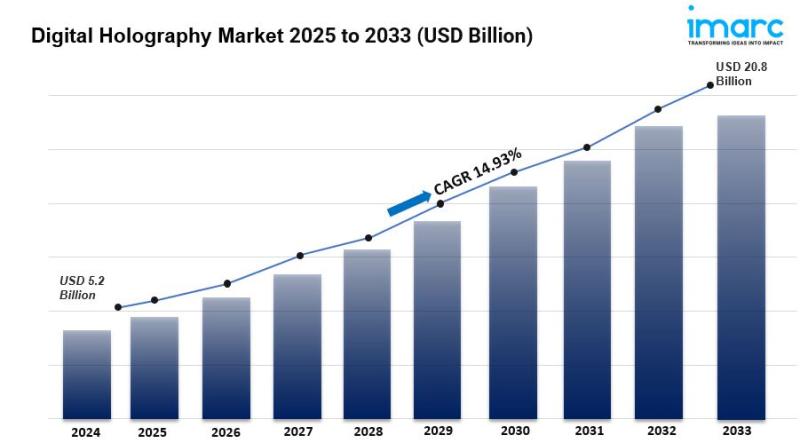
Digital Holography Market is Expected to Reach USD 20.76 Billion by 2033 | At CA …
Overview of the Digital Holography Market
The digital holography market is an emerging segment within the broader field of imaging technologies, leveraging holographic techniques to capture and reconstruct three-dimensional images. This technology is increasingly being adopted in various industries, including healthcare, telecommunications, and manufacturing, due to its ability to provide detailed visualizations and measurements.
The global digital holography market size was valued at USD 5.22 Billion in 2024. Looking forward, IMARC Group…
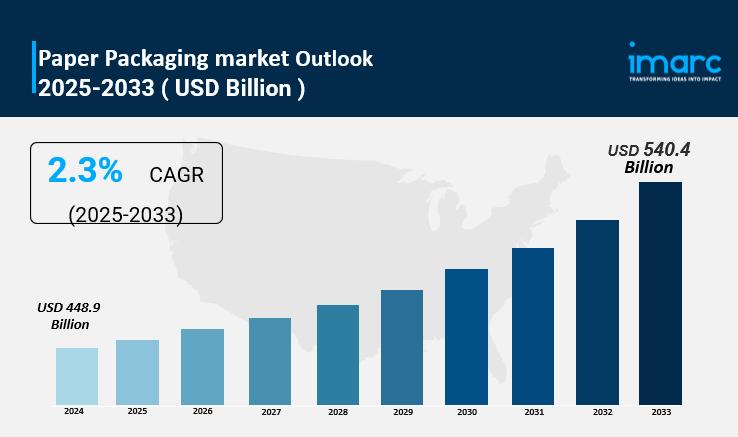
Paper Packaging is Expected to Grow USD 540.4 Billion by 2033 | At CAGR 2.3%
IMARC Group, a leading market research company, has recently released a report titled " Paper Packaging Market Size, Share, Trends and Forecast by Product, Material, Distribution Channel, Pricing, End-User, and Region, 2025-2033."The study provides a detailed analysis of the industry, including the Paper Packaging market size, share, trends, and growth forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Paper Packaging Market Overview
The…

India Luxury Car Market Size to Hit USD 1.98 Billion by 2034 | Expanding at 5.09 …
According to IMARC Group's report titled "India Luxury Car Market Size, Share, Growth | Forecast 2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
India Luxury Car Market Overview:
The India luxury car market was valued at USD 1.26 Billion in the base year 2025 and is projected to reach USD 1.98 Billion by 2034, growing at a compound annual growth rate (CAGR)…
More Releases for API
API Management Market Size, Trends Analysis 2032 by Key Vendors- Google, Cloud A …
USA, New Jersey: According to Verified Market Research analysis, the global API Management Market size was valued at USD 4.37 Billion in 2024 and is projected to reach USD 33.07 Billion by 2032, growing at a CAGR of 28.77% from 2026 to 2032.
What is the current outlook of the API Management Market and its expected growth potential?
The API Management Market is witnessing robust expansion due to the growing need…
Api 607 Vs API 608: A Comprehensive Comparison Guide Of Industrial Valve
Introduction: Why are API standards so important for industrial valves?
In high-risk industries such as oil and gas, chemicals and power, the safety and reliability of valves can directly affect the stability of production systems. The standards set by API (American Petroleum Institute) are the technical bible of industrial valves around the world. Among them, API 607 and API 608 are key specifications frequently cited by engineers and buyers.
This article will…
Vehicle API Market 2023 | Futuristic Technology- CarAPI, Caruso, One Auto API, A …
The Vehicle API market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The Vehicle API report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The Vehicle API research report also provides insights of different regions that are…
Face Recognition API Market Growth, Business Overview 2023, and Forecast to 2030 …
Facial recognition is a way of recognizing a human face through technology. A facial detection system uses biometrics to map facial features from a photograph or video. It compares information with a database of known faces to find a match. Moreover, the accuracy of facial recognition systems has improved way better in the last decade. Recent market developments and competitive strategies such as expansion, product launch, and development, partnership, merger,…
API Management Market Report 2018: Segmentation by Solution (API Portal, API Gat …
Global API Management market research report provides company profile for Akana, Inc. (U.S.), Apiary, Inc. (U.S.), Axway, Inc. (France), CA Technologies, Inc. (U.S.), Cloud Elements, Inc. (U.S.), Dell Boomi, Inc. (U.S.), DigitalML (U.S.), Fiorano Software, Inc. (U.S.), Google, Inc. (U.S.), Hewlett-Packard Enterprises Co. (U.S.), IBM Corporation (U.S.), Mashape Inc. (U.S.) and Others.
This market study includes data about consumer perspective, comprehensive analysis, statistics, market share, company performances (Stocks), historical…
Telecom API Market: OTT Service Providers Continue Cutting into Telecom API Prof …
The highly fragmented market of telecom API holds a staggering number of service providers and aggregators that are already offering their APIs to various telecom carriers. Alcatel Lucent, Apigee Corp., and Fortumo OU were the leading providers of telecom API from a global perspective in 2014. Telecom carriers have partnered with them and other prominent players in the past to launch APIs in the market.
According to Transparency Market Research’s latest…
