Press release
Duchenne Muscular Dystrophy Market Forecast to 2034: Regulatory Progress, FDA & EMA Decisions, Pipeline Therapies, Competitive Landscape, and Industry Players | DelveInsight
Major participants shaping the Duchenne Muscular Dystrophy (DMD) treatment ecosystem include Capricor Therapeutics, Santhera Pharmaceuticals in collaboration with ReveraGen Biopharma, Edgewise Therapeutics, FibroGen, Roche, Pfizer, Sarepta Therapeutics, Antisense Therapeutics, Italfarmaco, along with several emerging and established companies.The Duchenne Muscular Dystrophy market across the seven major markets (7MM) was valued at nearly USD 2.15 billion in 2023 and is projected to witness robust growth over the forecast period. This expansion is supported by higher treatment adoption rates, growing disease awareness, and the expected introduction of one-time gene therapies. Current therapeutic strategies focus on dystrophin gene replacement, exon skipping, muscle membrane stabilization, and reduction of inflammation.
In the United States, approved treatment options include EMFLAZA, VYONDYS 53, EXONDYS 51, AMONDYS 45, VILTEPSO, and the gene therapy ELEVIDYS, which continues to undergo regulatory review for full approval. In early 2024, Santhera Pharmaceuticals launched AGAMREE in Germany, marking its first commercial rollout.
Within the EU4 countries and the United Kingdom, corticosteroid therapies and TRANSLARNA dominate current treatment practices, while Japan relies primarily on VILTEPSO. Anticipated product launches from companies such as Santhera/ReveraGen, Taiho, FibroGen, Capricor, Italfarmaco, Antisense Therapeutics, and Sarepta are expected to further accelerate market growth, driven by rising prevalence and expanding access to innovative therapies globally.
DelveInsight's report titled "Duchenne Muscular Dystrophy Market Insights, Epidemiology, and Market Forecast - 2034" delivers an extensive analysis of the disease, combining historical data with future projections on epidemiology and market trends across the United States, EU4 (Germany, France, Italy, Spain), the United Kingdom, and Japan.
The DMD market is forecast to expand significantly throughout the study period, supported by increasing prevalence rates, heightened awareness, and the arrival of novel pipeline therapies spanning multiple stages of clinical development, which are expected to reshape the treatment landscape.
To gain deeper insights into the Duchenne Muscular Dystrophy market outlook, drug adoption patterns, treatment frameworks, and epidemiological trends, readers can explore the detailed market outlook report @ https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key Insights from the Duchenne Muscular Dystrophy Market Report
• The Duchenne Muscular Dystrophy market was valued at approximately USD 2.15 billion in 2023 and is expected to grow at a strong CAGR between 2020 and 2034.
• In December 2025, Capricor Therapeutics (NASDAQ: CAPR) announced positive topline outcomes from its pivotal Phase III HOPE-3 trial, evaluating Deramiocel, an investigational cell therapy for DMD.
• In September 2025, sDyne Therapeutics (NASDAQ: DYN) received Orphan Drug Designation from Japan's Ministry of Health, Labour and Welfare for DYNE-251, intended for DMD patients eligible for exon 51 skipping. The therapy is currently being studied in the global Phase I/II DELIVER trial.
• In June 2025, Cumberland Pharmaceuticals (NASDAQ: CPIX) presented encouraging Phase II FIGHT DMD trial results for ifetroban, an oral therapy targeting DMD-related cardiomyopathy, at the Parent Project Muscular Dystrophy conference, highlighting cardiac biomarker improvements and favorable pharmacokinetic data.
• In March 2025, Capricor Therapeutics announced that the FDA accepted its Biologics License Application for deramiocel to treat DMD cardiomyopathy, granting Priority Review with a PDUFA action date of August 31, 2025.
• Also in March 2025, Italfarmaco provided a detailed update on the regulatory progress and ongoing clinical trials for givinostat, its lead DMD therapy.
• In November 2024, Cumberland Pharmaceuticals received Orphan Drug and Rare Pediatric Disease Designations from the FDA for ifetroban in DMD-associated cardiomyopathy.
• In January 2024, Santhera Pharmaceuticals initiated commercial availability of AGAMREE (vamorolone) in Germany for DMD patients aged four years and above.
• The United States recorded approximately 17,000 prevalent DMD cases in 2023, with the highest burden observed in children aged 5-9 years, and prevalence is expected to rise through 2034.
• The US accounted for the largest share of DMD cases among the 7MM in 2023, with around 17,200 patients, a figure projected to increase steadily.
• In 2023, mutation distribution in the US included nearly 13,800 large mutations, 3,400 small mutations, and 1,700 point mutations, all expected to grow alongside rising prevalence.
• Among EU4 and the UK, the United Kingdom reported the highest DMD prevalence, followed by Germany and France, while Spain recorded the lowest numbers.
• Currently approved US therapies include EMFLAZA, VYONDYS 53, EXONDYS 51, AMONDYS 45, and VILTEPSO. In EU4 and the UK, steroid therapies and TRANSLARNA dominate, while Japan's approved treatment landscape is limited to VILTEPSO.
• Leading DMD developers include Italfarmaco, Antisense Therapeutics, Sarepta Therapeutics, Santhera/ReveraGen, Pfizer, FibroGen, Capricor Therapeutics, Roche, Edgewise Therapeutics, Wave Life Sciences, PepGen, Ultragenyx, and others.
• Prominent pipeline therapies include givinostat, ATL1102, SRP-9001, vamorolone, PF-06939926, pamrevlumab, CAP-1002, delandistrogene moxeparvovec, EDG-5506, WVE-N531, PGN-EDO51, and UX810.
• Gender-based epidemiology shows that DMD predominantly affects males, with extremely rare cases reported in females.
• In 2023, the 5-9 age group represented the largest patient segment, followed by ages 10-14, while DMD occurrence is rare in individuals over 30 years.
• The DMD market is expected to experience strong growth during the forecast period due to rising prevalence, improved diagnosis, and the introduction of late- and mid-stage pipeline therapies.
Request for Free Sample Report for Latest Duchenne Muscular Dystrophy Statistics: Duchenne Muscular Dystrophy Treatment Market - https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Duchenne Muscular Dystrophy Overview
Duchenne Muscular Dystrophy is a rare genetic disorder characterized by progressive muscle weakness and degeneration, primarily affecting young boys, with symptom onset typically between ages three and five. The condition is caused by mutations in the dystrophin gene, resulting in the absence or malfunction of dystrophin, a protein essential for muscle fiber stability.
Diagnosis typically involves genetic testing and, in some cases, muscle biopsy. While no cure exists, disease management includes physical therapy, orthopedic interventions, respiratory support, and pharmacological treatments such as corticosteroids. Emerging approaches, including gene therapy and exon-skipping technologies, offer significant promise for future care.
DMD follows a progressive course, often leading to loss of ambulation during adolescence and severe cardiopulmonary complications in adulthood. Advances in treatment and multidisciplinary care have significantly improved survival and quality of life, underscoring the importance of early diagnosis and comprehensive disease management.
Duchenne Muscular Dystrophy Epidemiology
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2020 to 2034. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. The epidemiology section also provides a detailed analysis of the diagnosed patient pool and future trends.
Duchenne Muscular Dystrophy Epidemiology Segmentation:
The Duchenne Muscular Dystrophy market report proffers epidemiological analysis for the study period 2020-2034 in the 7MM segmented into:
• Total Prevalence of Duchenne Muscular Dystrophy
• Prevalent Cases of Duchenne Muscular Dystrophy by severity
• Gender-specific Prevalence of Duchenne Muscular Dystrophy
• Diagnosed Cases of Episodic and Chronic Duchenne Muscular Dystrophy
Download the report to understand which factors are driving Duchenne Muscular Dystrophy epidemiology trends @ https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Duchenne Muscular Dystrophy Drugs Uptake and Pipeline Development Activities
The drugs uptake section focuses on the rate of uptake of the potential drugs recently launched in the Duchenne Muscular Dystrophy market or expected to get launched during the study period. The analysis covers Duchenne Muscular Dystrophy market uptake by drugs, patient uptake by therapies, and sales of each drug.
Moreover, the therapeutics assessment section helps understand the drugs with the most rapid uptake and the reasons behind the maximal use of the drugs. Additionally, it compares the drugs based on market share.
The report also covers the Duchenne Muscular Dystrophy Pipeline Development Activities. It provides valuable insights about different therapeutic candidates in various stages and the key companies involved in developing targeted therapeutics. It also analyzes recent developments such as collaborations, acquisitions, mergers, licensing patent details, and other information for emerging therapies.
Duchenne Muscular Dystrophy Competitive Landscape and Emerging Opportunities
• Givinostat (ITF2357): Italfarmaco
• ATL1102: Antisense Therapeutics
• SRP-9001: Sarepta Therapeutics
• Vamorolone: Santhera Pharmaceuticals/ReveraGen Biopharma
• PF06939926: Pfizer
• Pamrevlumab: FibroGen
• CAP-1002: Capricor Therapeutics
• Pamrevlumab: Fibrogen
• Delandistrogene moxeparvovec: Roche/Sarepta Therapeutics
• EDG 5506: Edgewise Therapeutics
• WVE N531: Wave Life Sciences Ltd
• PGN EDO51: PepGen
• UX810: Ultragenyx Pharmaceutical
Request for Sample Report @ Duchenne Muscular Dystrophy Companies and FDA Approvals - https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Duchenne Muscular Dystrophy Market Drivers and Growth Barriers
Key factors driving growth in the Duchenne Muscular Dystrophy market include:
• Rising disease awareness and earlier genetic diagnosis
• Expanding newborn screening initiatives
• Strong regulatory incentives such as Orphan Drug, Fast Track, and Priority Review designations
• Increasing investment in rare disease R&D
• Growing patient advocacy and trial participation
However, market expansion may be constrained by high therapy costs, limited mutation applicability, long-term safety uncertainties, and regulatory scrutiny of surrogate endpoints, particularly for gene and exon-skipping therapies.
Duchenne Muscular Dystrophy Future Outlook Through 2034
The Duchenne Muscular Dystrophy market is expected to witness substantial expansion through 2034, supported by late-stage pipeline advancements, geographic expansion into emerging markets, and evolving treatment paradigms that emphasize early intervention and combination therapy.
As innovation accelerates and competition intensifies, pricing strategies, reimbursement decisions, and long-term clinical outcomes will play a critical role in shaping market access and commercial success. Companies that demonstrate durable efficacy, cardiac benefit, and scalable manufacturing are likely to emerge as long-term leaders in the DMD space.
Scope of the Duchenne Muscular Dystrophy Market Report
• Study Period: 2020-2034
• Coverage: 7MM [The United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan]
• Key Duchenne Muscular Dystrophy Companies: Capricor Therapeutics (NASDAQ: CAPR), Santhera Pharmaceuticals (SWX: SANN), ReveraGen Biopharma (Private), Edgewise Therapeutics (NASDAQ: EWTX), FibroGen (NASDAQ: FGEN), Roche (SWX: ROG), Pfizer (NYSE: PFE), Sarepta Therapeutics (NASDAQ: SRPT), Antisense Therapeutics (ASX: ANP), Italfarmaco (Private), and others
• Key Duchenne Muscular Dystrophy Therapies: Givinostat (ITF2357), ATL1102, SRP-9001, Vamorolone, PF06939926, Pamrevlumab, CAP-1002, Pamrevlumab, Delandistrogene moxeparvovec, EDG 5506, WVE N531, PGN EDO51, UX810, and others
• Duchenne Muscular Dystrophy Therapeutic Assessment: Duchenne Muscular Dystrophy current marketed and Duchenne Muscular Dystrophy emerging therapies
• Duchenne Muscular Dystrophy Market Dynamics: Duchenne Muscular Dystrophy market drivers and Duchenne Muscular Dystrophy market barriers
• Competitive Intelligence Analysis: SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies
• Duchenne Muscular Dystrophy Unmet Needs, KOL's views, Analyst's views, Duchenne Muscular Dystrophy Market Access and Reimbursement
To know more about Duchenne Muscular Dystrophy companies working in the treatment market, visit @ Duchenne Muscular Dystrophy Therapies and Drugs - https://www.delveinsight.com/sample-request/duchenne-muscular-dystrophy-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Table of Contents
1. Duchenne Muscular Dystrophy Market Report Introduction
2. Executive Summary for Duchenne Muscular Dystrophy
3. SWOT analysis of Duchenne Muscular Dystrophy
4. Duchenne Muscular Dystrophy Patient Share (%) Overview at a Glance
5. Duchenne Muscular Dystrophy Market Overview at a Glance
6. Duchenne Muscular Dystrophy Disease Background and Overview
7. Duchenne Muscular Dystrophy Epidemiology and Patient Population
8. Country-Specific Patient Population of Duchenne Muscular Dystrophy
9. Duchenne Muscular Dystrophy Current Treatment and Medical Practices
10. Duchenne Muscular Dystrophy Unmet Needs
11. Duchenne Muscular Dystrophy Emerging Therapies
12. Duchenne Muscular Dystrophy Market Outlook
13. Country-Wise Duchenne Muscular Dystrophy Market Analysis (2020-2034)
14. Duchenne Muscular Dystrophy Market Access and Reimbursement of Therapies
15. Duchenne Muscular Dystrophy Market Drivers
16. Duchenne Muscular Dystrophy Market Barriers
17. Duchenne Muscular Dystrophy Appendix
18. Duchenne Muscular Dystrophy Report Methodology
19. DelveInsight Capabilities
20. Disclaimer
21. About DelveInsight
Contact Us:
Ankit Nigam
Manager Marketing
info@delveinsight.com
+14699457679
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Duchenne Muscular Dystrophy Market Forecast to 2034: Regulatory Progress, FDA & EMA Decisions, Pipeline Therapies, Competitive Landscape, and Industry Players | DelveInsight here
News-ID: 4360420 • Views: …
More Releases from DelveInsight Business Research
Panic Disorder Market Outlook Through 2032: Growing Worldwide Disease Burden and …
DelveInsight's latest report, "Panic Disorder Market Insights, Epidemiology, and Market Forecast-2032," presents an in-depth analysis of the Panic Disorder market, encompassing past performance, present dynamics, and future outlook across key global regions, including the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
This updated market forecast provides a detailed overview of the Panic Disorder landscape, offering actionable insights into revenue trends, prevalence rates, and shifts in…
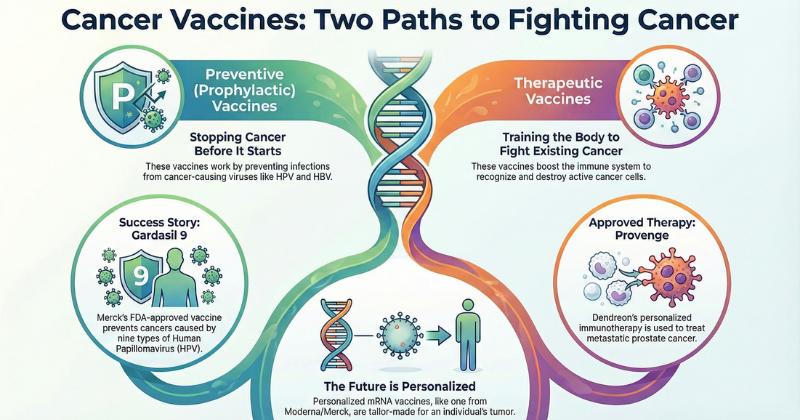
Cancer Vaccines Clinical Trial Outlook: 250+ Companies, 300+ Assets, and the Fut …
DelveInsight has released its newest strategic intelligence publication, "Cancer Vaccines - Competitive Landscape," providing an in-depth assessment of the global cancer vaccines ecosystem. The report evaluates more than 250 actively engaged companies and over 300 marketed and pipeline-stage assets, delivering a comprehensive view of innovation trends across preventive and therapeutic cancer vaccines. It incorporates advanced pipeline analytics, partnership evaluations, route of administration (ROA) analysis, molecular profiling, discontinued asset tracking, and…
Clostridium Difficile Infections Clinical Trials Outlook: 20+ Companies, 22+ Pip …
According to DelveInsight's evaluation, the global Clostridium difficile infections (Clostridium difficile infections) pipeline features more than 20 active pharmaceutical and biotechnology companies collectively advancing over 22 therapeutic candidates. These development programs span multiple clinical stages and are assessed across parameters such as clinical trial progress, therapeutic approaches, mechanisms of action, routes of administration, and recent developmental milestones.
DelveInsight's "Clostridium Difficile Infections Pipeline Insight" report delivers an in-depth overview of the current…
GLP-1 Agonists Market 2034: 7MM Insights, 6+ Active Companies, 5 Key Pipeline Dr …
DelveInsight's latest GLP-1 Agonists Market Insights Report provides a in-depth and forward-looking analysis of one of the most dynamic therapeutic classes shaping the future of metabolic disorder management. With expanding clinical trial activity, evolving regulatory pathways across the FDA, EMA, and PMDA, and a strong pipeline of next-generation candidates, GLP-1 agonists are redefining treatment approaches for type 2 diabetes, obesity, and other cardiometabolic conditions across the seven major markets (7MM).
The…
More Releases for Muscular
Limb Girdle Muscular Dystrophy Pipeline Insight 2025: Advancing Genetic and Rege …
DelveInsight's "Limb Girdle Muscular Dystrophy (LGMD) - Pipeline Insight, 2025" report provides a deep dive into the evolving therapeutic landscape of LGMD, a group of genetically inherited muscular disorders characterized by progressive weakness in the hip and shoulder muscles. With more than 25 active pipeline candidates targeting various LGMD subtypes, the field is witnessing a shift toward personalized gene therapies and precision treatments.
Recent advances have focused on gene replacement, exon…
Evolving Market Trends In The Spinal Muscular Atrophy Industry: Revolutionizing …
Our market reports now include the latest updates on global tariffs, trade impacts, and evolving supply chain dynamics.
What Is the Expected Spinal Muscular Atrophy Market Size During the Forecast Period?
Recent years have witnessed a swift expansion of the spinal muscular atrophy market size. The market is projected to surge from $3.53 billion in 2024 to $4.01 billion in 2025, escalating at a compound annual growth rate (CAGR) of 13.5%. The…
Duchenne Muscular Dystrophy Treatmcent Market
Global Duchenne Muscular Dystrophy Treatmcent Market Set for Robust Growth During Forecast Period
The global Duchenne Muscular Dystrophy Treatment Market is poised to witness significant growth at a high Compound Annual Growth Rate (CAGR) during the forecast period of 2023 to 2030. Duchenne muscular dystrophy (DMD) stands as a genetic disorder marked by progressive muscle degeneration and weakness, with therapeutics aimed at addressing the absence of dystrophin, a crucial protein in…
Becker Muscular Dystrophy Market Research Report 2032
DelveInsight's "Becker Muscular Dystrophy Market Insights, Epidemiology, and Market Forecast-2032" report delivers an in-depth understanding of the Becker Muscular Dystrophy (BMD), historical and forecasted epidemiology as well as the Becker Muscular Dystrophy (BMD) market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.
Becker Muscular Dystrophy (BMD) is an inherited muscle structure disorder that results in progressive deterioration of limbs, cardiac and skeletal muscles. However,…
Increasing cases of Spinal Muscular Atrophy will accelerate the Spinal Muscular …
Precision Business Insights published a research report on "Spinal Muscular Atrophy Management Market: By Type of Disease (Type-I, Type-II, Type-III, Type-IV), By Management (Gene Therapy, Drugs), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Others), and Geography- Global/Region/Country Forecast to 2027", The Global Muscular Atrophy Management Market is anticipated to grow at considerable level during forecast period 2021-2027. Spinal Muscular Atrophy Management Market was valued at USD 1.3 Billion in 2020…
Spinal Muscular Atrophy Market is Driven by Rising Number of People Affected wit …
The global spinal muscular atrophy market witnesses substantial competition with the presence of a considerable number of market participants of varying sizes, says Transparency Market Research (TMR) in a new market study.
Read Report Overview - https://www.transparencymarketresearch.com/spinal-muscular-atrophy-market.html
Savvy players are engaged in developing novel drug formulations, the success rate of which impacts market share, and trust of these companies among consumers. For such initiatives, large players are partnering with regional companies to…