Press release
Cancer Vaccines Clinical Trial Outlook: 250+ Companies, 300+ Assets, and the Future of Oncology Immunization, analyses DelveInsight
DelveInsight has released its newest strategic intelligence publication, "Cancer Vaccines - Competitive Landscape," providing an in-depth assessment of the global cancer vaccines ecosystem. The report evaluates more than 250 actively engaged companies and over 300 marketed and pipeline-stage assets, delivering a comprehensive view of innovation trends across preventive and therapeutic cancer vaccines. It incorporates advanced pipeline analytics, partnership evaluations, route of administration (ROA) analysis, molecular profiling, discontinued asset tracking, and commercial benchmarking tools, enabling stakeholders to assess competitive positioning and investment potential across the oncology vaccine landscape.Get instant access to key insights, pipeline snapshots, and competitive intelligence from DelveInsight's Cancer Vaccines Pipeline Report 2025 @ https://www.delveinsight.com/report-store/cancer-vaccines-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key Highlights from the Cancer Vaccines Market Report
• Prominent companies developing cancer vaccines include Imvax, Transgene (EPA: TNG), BrightPath Biotherapeutics, Amal Therapeutics, Enterome, Moderna (NASDAQ: MRNA), Vaccitech (NASDAQ: VACC), Ultimovacs ASA (OSE: ULTI), OSE Immunotherapeutics (EPA: OSE), IO Biotech (NASDAQ: IOBT), PDS Biotech (NASDAQ: PDSB), LIKANG Life Sciences, ISA Pharmaceuticals, Voltron Therapeutics, RNAImmune, AdaptVac, AVAX Technologies, Evaxion Biotech (NASDAQ: EVAX), YS Biopharma (NASDAQ: YSBP), Gritstone bio (NASDAQ: GRTS), BioNTech (NASDAQ: BNTX), Aston Science, Immunovative Therapies, SQZ Biotech (NASDAQ: SQZ), Checkmate Pharmaceuticals, Amphera, Virion Therapeutics, VAXIMM, Combined Therapeutics, Mendus (OMX: IMMU), Nykode Therapeutics (OSE: NYKD), AlphaVax, OncoQuest, Gradalis, Frame Therapeutics, VBI Vaccines (NASDAQ: VBIV), and others.
• Notable cancer vaccine candidates advancing across multiple indications and development stages include IGV 001, TG4050, BP1209, VTP-1100, ATP-128, EO2401, mRNA-4157, UV1, Tedopi, IO102, IO103, PDS0101, LK101, ISA101, ES2B C001, EVX-03, YS ON 001, MVax, GRANITE, NEO-PV-01, AST-301, AlloVax, SQZ-PBMC-HPV, Vidutolimod, autologous dendritic cell vaccines, VRON-0100, VXM-10, mRNA-based cancer vaccine research programs, Vididencel, VB 10.16, AVX701, P53-SLP vaccine, Oregovomab, EO2040, Vigil EWS, FRAME-001 personalized vaccine, VBI-1901, and others.
• Growth of the global cancer vaccines market is primarily driven by the rising incidence of cancers such as breast, prostate, cervical, and lung cancer, alongside increasing public and private investment, strong government funding initiatives, expanding use of vaccines in combination regimens, growing demand for immunotherapy-based prevention, higher awareness of vaccine benefits, increased prevalence of HPV infections, and the introduction of next-generation vaccine platforms.
• Moderna, in collaboration with Merck, continues to lead the field with intismeran autogene (mRNA-4157/V940), a personalized mRNA cancer vaccine. In January 2026, long-term follow-up data from a Phase 2 study in high-risk melanoma showed a sustained reduction in recurrence or death when the vaccine was combined with pembrolizumab. The program has since advanced into Phase 3, with additional trials expanding into non-small cell lung cancer, renal cell carcinoma, and bladder cancer.
• Evaxion A/S has reported encouraging results for its AI-designed personalized cancer vaccine EVX-01 in metastatic melanoma. During 2024, the Phase 2 trial completed patient dosing, and updated data presented in October 2025 demonstrated a high objective response rate along with durable immune responses. Additional immune biomarker findings were shared later in November 2025, supporting the vaccine's differentiated mechanism.
• In early-stage development, Everest Medicines initiated first-in-human studies of its personalized mRNA cancer vaccine EVM16. The first patient was dosed in March 2025, marking a key milestone for the company's entry into the therapeutic cancer vaccine space, with initial efforts focused on solid tumors.
• Anixa Biosciences, working with the Cleveland Clinic, is developing an alpha-lactalbumin (aLA) breast cancer vaccine designed primarily for prevention and early intervention. The Phase 1 trial completed its final patient visit in October 2025, and full safety and immunogenicity results were scheduled for presentation in December 2025, positioning the program for potential next-stage development.
• On August 24, 2025, Columbia University announced a clinical study evaluating the 9-valent HPV (9vHPV) vaccine in children, adolescents, and young women living with HIV in Eswatini. The study aims to compare the immunogenicity of a two-dose 9vHPV regimen in HIV-positive individuals aged 9-26 years with a three-dose regimen administered to HIV-negative young women.
Access detailed insights on 300+ assets, key players, and late-stage opportunities-download the complete pipeline report today @ https://www.delveinsight.com/sample-request/cancer-vaccines-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Cancer Vaccines Explained: A Transformative Approach in Oncology
The rapid advancement of cancer immunotherapy has reshaped treatment paradigms across multiple tumor types. While immune checkpoint inhibitors and monoclonal antibodies have produced significant clinical benefits in selected patient populations, challenges such as variable response rates and immune evasion persist. Cancer vaccines address these gaps by activating the immune system to specifically recognize and destroy tumor-associated antigens (TAAs) or patient-specific neoantigens through controlled immune priming.
Unlike antibody-driven therapies that mainly modulate immune checkpoints, cancer vaccines establish durable immune memory and sustained effector responses. By reshaping tumor-immune interactions, vaccines are emerging as precision immuno-oncology tools with applications in both cancer prevention and active treatment.
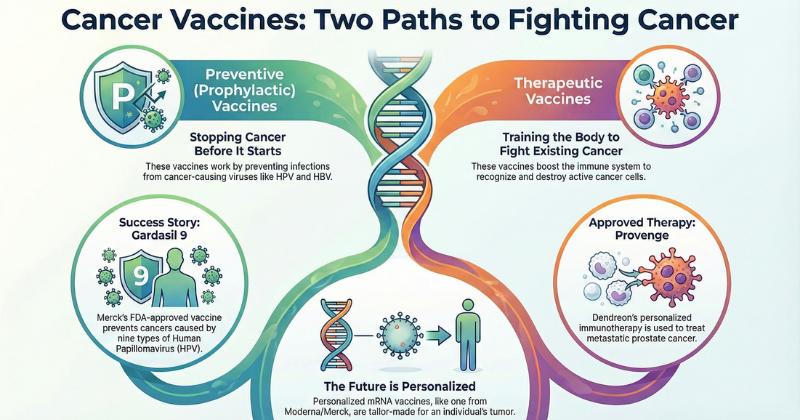
Preventive and Therapeutic Cancer Vaccine Categories
Cancer vaccines are broadly classified into two major types:
• Preventive (Prophylactic) Vaccines:
These vaccines are designed to block virus-induced cancer development. HPV and HBV vaccines represent the most successful preventive oncology interventions to date. The FDA-approved Gardasil 9 (Merck) remains the standard of care for preventing cervical, vaginal, anal, and oropharyngeal cancers, offering strong population-level evidence for vaccination-based cancer prevention.
• Therapeutic Vaccines:
Therapeutic cancer vaccines aim to stimulate immune responses against existing tumors. The field is increasingly focused on personalized neoantigen vaccines utilizing mRNA, DNA, peptide-based platforms, dendritic cells, and viral vectors. These therapies are frequently combined with immune adjuvants, cytokines, or checkpoint inhibitors to amplify T-cell activation and antigen presentation.
Discover which cancer vaccine programs are advancing fastest and where strategic investments are heading-request your copy now. @ https://www.delveinsight.com/report-store/cancer-vaccines-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Approved Cancer Vaccine Therapies
Dendreon Corporation
Dendreon is a biotechnology company recognized for developing Provenge (sipuleucel-T), an autologous cellular immunotherapy for prostate cancer. The therapy is manufactured using a patient's own immune cells, particularly dendritic cells, which are exposed ex vivo to the proprietary PAP-GM-CSF fusion protein. The company's technology platform allows for substitution of disease-specific antigens, enabling potential expansion into additional therapeutic areas.
Provenge (Sipuleucel-T)
Provenge is a personalized cellular immunotherapy targeting prostatic acid phosphatase (PAP), expressed in approximately 95% of prostate cancers. Each treatment course is individually prepared for the patient. In the IMPACT Phase III trial, Provenge demonstrated a median overall survival improvement of 4.1 months in metastatic prostate cancer. The FDA approved the therapy in April 2010 for asymptomatic or minimally symptomatic metastatic hormone-refractory prostate cancer.
Merck & Co., Inc.
Merck (MSD outside the U.S. and Canada) is a global biopharmaceutical leader focused on oncology, infectious diseases, and vaccine innovation. The company continues to advance immunology-driven therapies and remains at the forefront of cancer prevention through vaccination science.
HPV Vaccine Portfolio
Merck's HPV vaccine targets multiple oncogenic and non-oncogenic HPV strains, including types 6, 11, 16, 18, 31, 33, 45, 52, and 58. It is approved for preventing precancerous lesions and cancers of the cervix, vagina, anus, and genital warts. In males aged 9-45 years, it is also indicated for preventing HPV-related anal, head and neck, and oropharyngeal cancers.
Emerging Cancer Vaccine Therapies
OSE Immunotherapeutics
OSE Immunotherapeutics focuses on immunotherapy development across oncology and autoimmune diseases, with platforms spanning T-cell vaccines and myeloid-targeted programs. Its most advanced candidate, Tedopi, is a neoepitope-based therapeutic vaccine.
Tedopi (OSE-2101)
Tedopi is under development for non-small cell lung cancer (NSCLC) and contains ten optimized neoepitopes derived from five tumor-associated antigens. The vaccine stimulates cytotoxic T-cell responses and has received FDA Orphan Drug Designation. In Europe, it is positioned as a personalized treatment for HLA-A2-positive NSCLC patients.
IO Biotech
IO Biotech is a clinical-stage biotechnology company developing immune-modulating cancer vaccines using its proprietary T-win® platform. Its lead candidate, IO102-IO103, is advancing through late-stage clinical trials.
IO102-IO103
This dual vaccine targets immune suppression mediated by IDO and PD-L1 pathways. Currently in Phase III trials for melanoma, it is also being explored in earlier-stage studies across multiple tumor types. The therapy combines immune activation with direct elimination of suppressive immune cells, delivering robust anti-tumor effects with favorable tolerability.
PDS Biotech
PDS Biotech leverages its Versamune® platform to enhance antigen delivery and type I interferon activation, generating strong cytotoxic T-cell and antibody responses.
PDS0101
PDS0101 is an investigational HPV-targeted immunotherapy administered subcutaneously, often in combination regimens. Clinical studies have shown significant immune activation, tumor regression, and delayed disease progression without added toxicity. The candidate is currently being evaluated across four Phase II trials.
Explore Phase-wise pipeline analysis, partnering activity, and emerging platforms shaping the future of cancer vaccines get the report @ https://www.delveinsight.com/sample-request/cancer-vaccines-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Strategic Collaborations Accelerating Market Growth
The cancer vaccine market continues to see robust partnership activity, including:
• February 2023: Personalis expanded its collaboration with Moderna to support mRNA-4157/V940 development using NeXT Platform® analytics.
• January 2023: GreenLight Biosciences partnered with EpiVax Therapeutics to commercialize personalized mRNA cancer vaccines.
• November 2022: IO Biotech broadened its clinical collaboration with Merck to study IO102-IO103 in combination with KEYTRUDA®.
• October 2022: Merck exercised its option to co-commercialize mRNA-4157/V940 with Moderna.
• March 2022: NEC OncoImmunity acquired VAXIMM's neoantigen DNA vaccine platform.
Late-Stage and Marketed Cancer Vaccines
Provenge (Dendreon Corporation)
• Modality: Autologous cellular immunotherapy
• Indication: Metastatic hormone-refractory prostate cancer
• Regulatory Status: FDA approved (2010)
• Clinical Benefit: 4.1-month median survival improvement
Gardasil 9 (Merck & Co.)
• Modality: 9-valent recombinant HPV vaccine
• Coverage: HPV-driven cervical, anal, oropharyngeal, and genital cancers
• Regulatory Expansion: Head and neck cancer prevention added in 2020
Request for Sample report @ https://www.delveinsight.com/sample-request/cancer-vaccines-competitive-landscape?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key Late-Stage Pipeline Candidates
• Tedopi (OSE Immunotherapeutics): Phase III neoepitope peptide vaccine
• IO102-IO103 (IO Biotech): Phase III immune-modulating vaccine (melanoma)
• PDS0101 (PDS Biotech): Phase II HPV-targeted combination therapy
These candidates demonstrate strong immunogenic profiles and represent leading contenders in next-generation cancer vaccine development.
Report Coverage and Strategic Insights
• Global geographic scope
• ROA analysis: intradermal and subcutaneous delivery
• Leading companies profiled: Moderna, Merck, OSE Immunotherapeutics, IO Biotech, PDS Biotech, Transgene, Vaccitech, Imvax, Ultimovacs ASA, and others
• In-depth evaluation of DNA, mRNA, viral vector, peptide, and dendritic cell vaccine platforms
Key Questions Answered
• How many companies are actively developing cancer vaccines?
• What is the phase-wise distribution of pipeline assets?
• Which platforms dominate personalized neoantigen vaccine development?
• What collaboration and licensing trends define recent deal activity?
• Where combination therapy activity is most concentrated globally?
Contact Us:
Ankit Nigam
Manager Marketing
info@delveinsight.com
+14699457679
https://www.delveinsight.com/consulting
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Cancer Vaccines Clinical Trial Outlook: 250+ Companies, 300+ Assets, and the Future of Oncology Immunization, analyses DelveInsight here
News-ID: 4360370 • Views: …
More Releases from DelveInsight Business Research
Clostridium Difficile Infections Clinical Trials Outlook: 20+ Companies, 22+ Pip …
According to DelveInsight's evaluation, the global Clostridium difficile infections (Clostridium difficile infections) pipeline features more than 20 active pharmaceutical and biotechnology companies collectively advancing over 22 therapeutic candidates. These development programs span multiple clinical stages and are assessed across parameters such as clinical trial progress, therapeutic approaches, mechanisms of action, routes of administration, and recent developmental milestones.
DelveInsight's "Clostridium Difficile Infections Pipeline Insight" report delivers an in-depth overview of the current…
GLP-1 Agonists Market 2034: 7MM Insights, 6+ Active Companies, 5 Key Pipeline Dr …
DelveInsight's latest GLP-1 Agonists Market Insights Report provides a in-depth and forward-looking analysis of one of the most dynamic therapeutic classes shaping the future of metabolic disorder management. With expanding clinical trial activity, evolving regulatory pathways across the FDA, EMA, and PMDA, and a strong pipeline of next-generation candidates, GLP-1 agonists are redefining treatment approaches for type 2 diabetes, obesity, and other cardiometabolic conditions across the seven major markets (7MM).
The…

WEGOVY (Semaglutide) Market Size, Forecast, and Drug Insight Report Highlighting …
DelveInsight, a leading life science market research and business consulting firm, has announced the release of its latest in-depth report titled "WEGOVY (Semaglutide), Market Size, Forecast, and Drug Insight - 2032." The report delivers a detailed and data-driven analysis of WEGOVY (Semaglutide) as a treatment for obesity across the seven major markets (7MM), including the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan, covering the…

HR Positive/ HER2 Negative Breast Cancer Pipeline 2025: Key Developments, Emergi …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, HR Positive/ HER2 Negative Breast Cancer pipeline constitutes 50+ key companies continuously working towards developing 53+ HR Positive/ HER2 Negative Breast Cancer treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
"HR Positive/ HER2 Negative Breast Cancer Pipeline Insight, 2025" report by DelveInsight outlines comprehensive insights into the present clinical…
More Releases for Vaccine
Adult Vaccines Market By Type (Preventive Vaccine, Therapeutic Vaccine)
Global Adult Vaccines Market 2022 Research Report initially provides a basic overview of the industry that covers definition, applications and manufacturing technology, post which the report explores into the international players in the market.
Download Sample PDF at https://www.theinsightpartners.com/sample/TIPRE00007244/?utm_source=OpenPR&utm_medium=10379
Key Players Analysis:
AstraZeneca
Bharat Biotech
Dynavax Technologies Corporation
GlaxoSmithKline
Johnson & Johnson
Merck and Co
Novartis
Pfizer
Sanofi Pasteur
Serum Institute of India
The report covers key developments in the Adult Vaccines market as organic and inorganic growth strategies. Various companies are focusing…
Flu Vaccine (Influenza Vaccine) Market: A look into the future of the “Univers …
Latest update on Flu Vaccine (Influenza Vaccine) Market Analysis Report published with an SMI, the industry growth analysis, and Projection by 2021-2028. This report is highly predictive as it holds the overall market analysis of the topmost companies in the Flu Vaccine (Influenza Vaccine) industry. With the classified Flu Vaccine (Influenza Vaccine) market research based on various growing regions, this report provides leading players' portfolios along with sales, growth, market…
Vaccine Market
Rise in the prevalence of infectious diseases, increase in immunization programs across the globe, and surge in R&D activities to develop new vaccine drive the growth of the global vaccine market.
Global Vaccines Market was pegged at $32.46 billion in 2019, and is anticipated to hit $54.15 billion by 2027, registering a CAGR of 6.6% from 2020 to 2027. The report provides an in-depth analysis of the top investment pockets, top…
US Flu Vaccine [Influenza Vaccine] Market Research Report 2020
DPI Research offers a latest published report on “Influenza Vaccines in the United States – Market Size, Trends, Opportunities and Growth Potential” delivering key insights and providing a competitive advantage to clients through a detailed report. This report focuses on the key US flu vaccines market players, to define, describe and analyze the value, market share, market competition landscape, SWOT analysis and development plans in next few years. To analyze…
Measles Vaccine
Measles Vaccine Market describes its growth, size, share, Forecast and trends to 2025
Measles Vaccine Market Production and Demand Analysis 2019 to 2025
Measles Vaccine Market 2019 Manufacturing Analysis and Development Forecast 2025
Measles Vaccine Market 2019: Recent Study Including Growth Factors, Regional Drivers, Forecast 2025
Measles Vaccine Market to Insight By 2025: Top Key Vendors
The global Measles Vaccine market is valued at million US$ in 2018 and will reach million US$ by the…
Monovalent vaccine segment to accumulate maximum Paediatric Vaccine Market share
According to a new report published by Future Market Insights titled “Paediatric Vaccine Market: Global Industry Analysis & Opportunity Assessment, 2016 – 2026”, in terms of revenue, the global paediatric vaccine market is expected to increase at 12.2% CAGR during the forecast period 2016-2026. The global paediatric vaccine market is expected to reach US$ 27.97 Bn in 2016.
Paediatric vaccine market is a billion dollar market accounting for a substantial proportion…