Press release
Liver Cancer Clinical Trials & Drug Pipeline Report: 75+ Therapies, 70+ Companies Analyzed, analyses DelveInsight
Liver cancer companies are Arcus Biosciences (NASDAQ: RCUS), Tvardi Therapeutics (NASDAQ: TVRD), GlaxoSmithKline (NYSE: GSK / LSE: GSK), AVEO Pharmaceuticals (formerly NASDAQ: AVEO), Teclison, Epizyme (formerly NASDAQ: EPZM), Sirnaomics (HKEX: 9877), Coherus Biosciences (NASDAQ: CHRS), Sinocelltech Ltd., Qurient Co. (KOSDAQ: 066820), Hoffmann-La Roche (SIX: ROG / OTC: RHHBY), Can-Fite BioPharma (NYSE American: CANF), Omega Therapeutics (NASDAQ: OMG), Bristol-Myers Squibb (NYSE: BMY), moreDelveInsight's "Liver Cancer Pipeline Insights 2025" delivers an in-depth overview of the liver cancer pipeline ecosystem, profiling more than 70 companies and 75+ therapeutic candidates under development. The report thoroughly examines liver cancer drug profiles across both clinical and preclinical stages. It also evaluates the therapeutic landscape by product category, development phase, route of administration, and molecular class, while additionally identifying inactive and discontinued assets within the pipeline.
Stay informed with cutting-edge developments! Access detailed Liver Cancer Pipeline Report to uncover emerging therapies, leading companies, and the future direction of liver cancer treatment @ Liver Cancer Pipeline Outlook Report - https://www.delveinsight.com/sample-request/liver-cancer-pipeline-insight [https://www.delveinsight.com/sample-request/liver-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Key Highlights from the Liver Cancer Pipeline Report
* In January 2026, Adjuvant pembrolizumab (Keytruda) failed to improve recurrence-free survival (RFS) in patients with hepatocellular carcinoma (HCC) following curative-intent resection or local ablation, according to results from the phase 3 KEYNOTE-937 trial presented at the 2026 ASCO Gastrointestinal Cancers Symposium in San Francisco. At a median follow-up of 50.7 months, RFS outcomes were similar between the pembrolizumab and placebo arms, with identical 48-month RFS rates of 50% and no overall survival advantage observed. The findings highlight the lack of an effective adjuvant immunotherapy option in early-stage HCC and underscore the ongoing unmet need to reduce post-treatment recurrence risk.
* In January 2026, Scientists at the Cancer Research UK Scotland Institute have identified a key protein, NPM1, linked to the growth of bowel and liver cancers, opening the door to safer and more targeted treatments. Published in Nature Genetics, the study reveals how genetic faults in the WNT signalling pathway drive excess NPM1 production, helping cancer cells survive and multiply. Researchers believe blocking NPM1 could halt tumour growth without damaging healthy tissue. As bowel and liver cancer cases continue to rise, particularly among younger adults, this discovery offers new hope for patients with limited treatment options and may have broader implications for other cancer types.
* In January 2026, Researchers at the Johns Hopkins Kimmel Cancer Center and St. Jude Children's Research Hospital have reported encouraging early results from the first clinical trial of a therapeutic vaccine for fibrolamellar carcinoma (FLC), one of the rarest and most difficult-to-treat liver cancers. Published in Nature Medicine, the Phase I study evaluated a vaccine targeting the DNAJB1-PRKACA fusion protein, a genetic hallmark present in nearly all FLC tumors. Among evaluable patients with unresectable disease, 75% achieved disease control, with several showing deep and durable responses. The vaccine was well tolerated and, in some cases, enabled patients to regain eligibility for surgery, offering new hope in a disease with no FDA-approved therapies.
* On Dec. 18, BeOne Medicines Ltd. announced that the FDA granted fast track designation to BGB-B2033, a GPC3x4-1BB bispecific antibody, for the treatment of adult patients with hepatocellular carcinoma whose disease has progressed on or after prior systemic therapy.
* August 2025: Hoffmann-La Roche initiated a Phase Ib/II clinical trial in patients with advanced liver cancers. The adaptive study design allows for the addition of new treatment arms, closure of arms with limited efficacy or safety concerns, adjustments to patient populations, and inclusion of new cohorts with other advanced primary liver cancer subtypes.
* July 2025: Bristol-Myers Squibb launched a clinical study assessing the safety and efficacy of relatlimab combined with nivolumab in patients with advanced liver cancer who are immunotherapy-naive but previously treated with tyrosine kinase inhibitors.
* DelveInsight's analysis highlights a highly competitive and active liver cancer pipeline, with over 70 organizations developing more than 75 investigational therapies.
* Key players in the liver cancer landscape include Arcus Biosciences, Yiviva, Virogin Biotech, Tvardi Therapeutics, GlaxoSmithKline, TORL Biotherapeutics, AVEO Pharmaceuticals, Teclison, Epizyme, Sirnaomics, Coherus Biosciences, Sinocelltech Ltd., Qurient Co., Hoffmann-La Roche, Can-Fite BioPharma, Omega Therapeutics, Novita Pharmaceuticals, Bristol-Myers Squibb, and others.
* Notable liver cancer therapies in development include Atezolizumab, Bevacizumab, Tiragolumab, OH2 Injection, MTL-CEBPA, Sorafenib 200 mg, Lipiodol, Pemetrexed, Exatecan Mesylate, Brivanib, among others.
Explore how the liver cancer treatment landscape is transforming. Gain deeper insights into novel assets and clinical progress through DelveInsight's comprehensive pipeline evaluation @ Liver Cancer Clinical Trials and Studies [https://www.delveinsight.com/sample-request/liver-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Liver Cancer Emerging Drugs Profile
- Namodenoson: Can-Fite BioPharma
Namodenoson is an orally administered small-molecule agent, also known as Cl-IB-MECA, that selectively targets the A3 adenosine receptor (A3AR). Its mechanism involves modulation of the NF-B and Wnt signaling pathways, triggering tumor cell apoptosis. Additionally, the drug exerts protective effects by suppressing NF-B-mediated signaling, thereby preventing excessive apoptosis.
Namodenoson has demonstrated strong anti-tumor activity - particularly in hepatocellular carcinoma - along with anti-inflammatory effects in preclinical liver inflammation models. Favorable safety outcomes have been observed in preclinical studies as well as Phase I and II clinical trials. The therapy is currently undergoing Phase III clinical development for advanced liver cancer.
- YIV-906: Yiviva
YIV-906 (also known as PHY906 or KD018) is a proprietary, cGMP-grade botanical formulation derived from four medicinal herbs rooted in traditional Chinese medicine. Designed as a platform oncology therapy, YIV-906 is intended for use alongside chemotherapy, immunotherapy, and radiation across multiple cancer indications. The compound has been shown to enhance immune activity within the tumor microenvironment, protect gastrointestinal tissues by suppressing inflammatory pathways (including IL-6, NF-B, COX-2, and iNOS), and stimulate intestinal repair via Wnt pathway activation. Preclinical data suggest synergistic anti-tumor effects when combined with sorafenib in hepatocellular carcinoma, with encouraging early clinical signals in liver, pancreatic, colorectal, and rectal cancers. YIV-906 is currently in Phase II clinical trials for liver cancer.
- TTI-101: Tvardi Therapeutics
TTI-101 is an orally available small-molecule inhibitor targeting STAT3, a transcription factor implicated in cancer progression, inflammation, and fibrosis. Preclinical studies have demonstrated favorable pharmacokinetics, potent inhibition of STAT3 phosphorylation, and significant tumor growth suppression in multiple cancer models. The therapy has advanced into Phase II clinical evaluation.
- STP707: Sirnaomics
STP707 is an RNA interference-based therapeutic candidate in early-stage development for solid tumors, including liver cancer. Administered intravenously, it simultaneously inhibits TGF-1 and COX-2, utilizing Sirnaomics' proprietary PNP delivery platform. The drug is currently being assessed in a Phase I clinical trial.
- BST02: BioSyngen
BST02 is an adoptive cell therapy derived from the expansion of a patient's own tumor-infiltrating lymphocytes (TILs). Designed to address all forms of liver cancer, BST02 offers advantages over conventional TIL therapies, including cryopreservation for logistical flexibility and reduced dependence on high-dose interleukin-2. The therapy is currently undergoing Phase I clinical testing.
Insights Offered by the Liver Cancer Pipeline Report
* Comprehensive profiling of companies developing liver cancer therapies, including the total number of assets per organization
* Classification of therapeutic candidates across early, mid, and late stages of development
* Coverage of both active and inactive (discontinued or dormant) pipeline programs
* Segmentation of drugs by development stage, administration route, molecular class, therapeutic modality, target, and mechanism of action
* In-depth evaluation of strategic collaborations, licensing deals, and funding activities shaping future market growth
Gain a strategic view of the most recent innovations shaping the liver cancer pipeline. Explore DelveInsight's expert-led analysis today @ Liver Cancer Unmet Needs [https://www.delveinsight.com/sample-request/liver-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Liver Cancer Companies
Arcus Biosciences, Yiviva, Virogin Biotech, Tvardi Therapeutics, GlaxoSmithKline, TORL Biotherapeutics, AVEO Pharmaceuticals, Teclison, Epizyme, Sirnaomics, Coherus Biosciences, Sinocelltech Ltd., Qurient Co., Hoffmann-La Roche, Can-Fite BioPharma, Omega Therapeutics, Novita Pharmaceuticals, Bristol-Myers Squibb, and others.
Therapeutic Assessment by Route of Administration (ROA)
Pipeline therapies are categorized based on administration routes, including:
* Intra-articular
* Intraocular
* Intrathecal
* Intravenous
* Oral
* Parenteral
* Subcutaneous
* Topical
* Transdermal
Therapeutic Assessment by Molecule Type
Liver cancer pipeline assets are segmented into:
* Oligonucleotides
* Peptides
* Small molecules
Download DelveInsight's latest report to uncover strategic insights into upcoming liver cancer therapies and key industry developments @ Liver Cancer Competitive Landscape [https://www.delveinsight.com/sample-request/liver-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Scope of the Liver Cancer Pipeline Report
* Geographical Coverage: Global
* Companies Covered: Arcus Biosciences, Yiviva, Virogin Biotech, Tvardi Therapeutics, GlaxoSmithKline, TORL Biotherapeutics, AVEO Pharmaceuticals, Teclison, Epizyme, Sirnaomics, Coherus Biosciences, Sinocelltech Ltd., Qurient Co., Hoffmann-La Roche, Can-Fite BioPharma, Omega Therapeutics, Novita Pharmaceuticals, Bristol-Myers Squibb, and others
* Therapies Covered: Atezolizumab, Bevacizumab, Tiragolumab, OH2 Injection, MTL-CEBPA, Sorafenib 200 mg, Lipiodol, Pemetrexed, Exatecan Mesylate, Brivanib, and others
* Therapeutic Modalities: Monotherapy, combination therapy, and mono/combination approaches
* Development Stages: Discovery, preclinical, Phase I, Phase II, and Phase III
Which companies are shaping the future of liver cancer drug development? Discover the leaders and breakthrough therapies in DelveInsight's exclusive Liver Cancer Pipeline Report - access it now @ Liver Cancer FDA Approvals and Companies [https://www.delveinsight.com/sample-request/liver-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing end to end comprehensive solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=liver-cancer-clinical-trials-drug-pipeline-report-75-therapies-70-companies-analyzed-analyses-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting/partner-identification-services
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Liver Cancer Clinical Trials & Drug Pipeline Report: 75+ Therapies, 70+ Companies Analyzed, analyses DelveInsight here
News-ID: 4358830 • Views: …
More Releases from ABNewswire
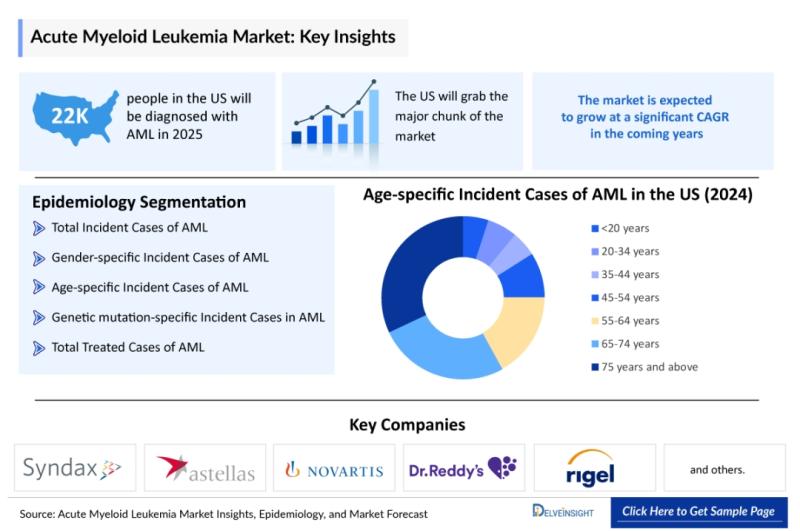
Acute Myeloid Leukemia Market Forecast to 2034: 7MM Opportunity, Strong CAGR, 43 …
Robust R&D activity, breakthrough regulatory designations, and precision oncology approaches are driving meaningful change in AML care and commercial dynamics.
The Acute Myeloid Leukemia (AML) market is expected to grow at a strong CAGR through 2034 across the US, EU4, UK, and Japan, driven by innovation in targeted therapies and rising disease incidence. In 2023, the US dominated the AML treatment market, with total 7MM incidence reaching ~43,500 cases, the highest…

NM-8074 (Ruxoprubart) Sales Forecast Signals Strong Long-Term Growth Potential A …
NM-8074 (ruxoprubart) sales market analysis provides in-depth insights into NM-8074 annual sales, market size, and growth forecast across the 7MM through 2034. The report evaluates NM-8074 sales potential by indication, pricing dynamics, competitive landscape, and regulatory milestones, highlighting commercial opportunities in complement-mediated diseases such as PNH, aHUS, and dermatomyositis.
DelveInsight is pleased to announce the release of its in-depth industry report titled "NM-8074 Sales Forecast, and Market Size Analysis - 2034",…

Paltusotine Sales Forecast Signals Significant Market Expansion Driven by Oral I …
The Paltusotine market is poised for significant expansion following FDA approval as the first once-daily oral therapy for acromegaly. Driven by strong physician adoption, growing patient preference for oral alternatives, and lifecycle expansion opportunities, Paltusotine annual sales are expected to rise steadily across the 7MM. The Paltusotine sales market forecast highlights robust growth, supported by competitive differentiation, global commercialization strategies, and more.
The acromegaly treatment landscape is undergoing a meaningful shift…
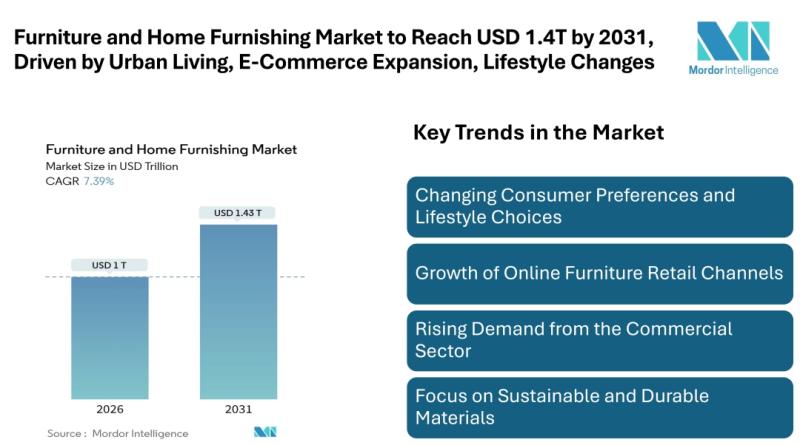
Furniture and Home Furnishing Market to Reach USD 1.43 trillion by 2031, Driven …
Mordor Intelligence has published a new report on furniture and home furnishing market offering a comprehensive analysis of trends, growth drivers, and future projections.
Furniture and Home Furnishing Market Overview:
According to Mordor Intelligence, the furniture and home furnishing market size [https://www.mordorintelligence.com/industry-reports/furniture-and-home-furnishing?utm_source=abnewswire] stands at USD 1.00 trillion in 2026 and is projected to reach USD 1.43 trillion by 2031 at a 7.39% CAGR, reflecting a clear acceleration from the 2019 to 2024…
More Releases for Cancer
Cancer Therapeutics Market New Business Opportunities to Hit $180.19 billion by …
Surge in geriatric population and rise in the number of collaborations & partnerships to facilitate drug development are the key drivers of the global cancer therapeutics market. In addition, heavy inflow of investment in R&D activities has enhanced the development of cancer therapeutics. Furthermore, favorable government regulations for cancer therapeutics and surge in cancer prevalence boost the market. The high demand for personalized medicine along with high potential of emerging…
Global Cancer Diagnostics Market Size, Trends & Growth Opportunity By Applicatio …
Cancer diagnostics is a process of detecting various proteins, biomarkers and certain symptoms that result in the detection of presence of cancerous tumour in patients. Detection of certain proteins and biomarkers which are prevalent in cancer disorder thereby results in diagnosis process. Cancer diagnostics includes usage of certain technology and devices for detection purpose.
Increase in incidence of target diseases like cancer is key driving factor which is expected to boost…
2019-2027 Oncology Nutrition Market is driven by Major Cancer Type - Head and Ne …
The "Global Oncology Nutrition Market Analysis to 2027" is a specialized and in-depth study with a special focus on the global market trend analysis. The report aims to provide an overview of oncology nutrition market with detailed market segmentation by cancer type and geography. The global oncology nutrition market is expected to witness high growth during the forecast period. The report provides key statistics on the market status of the…
Cancer Immunotherapy Market 2018 To 2025 - SWOT Analysis By Global Industry Reve …
The Cancer Immunotherapy Market research report provided by Crystal Market Research (CMR) is the most detailed study about Cancer Immunotherapy Market that is estimated to grow at a tremendous rate over the forecast period 2018-2025. This report contains precise and updated insights in respect with the leading market players and prevailing regions of the business.
Cancer Immunotherapy Market By Product and Cancer Type - Global Industry Analysis And Forecast To 2025:…
Oncology Nutrition Market by Cancer Type Breast Cancer, Liver Cancer, Lung Cance …
Lunch of new products for nutrition of oncology patients is expected to drive the oncology nutrition market growth. For instance, in 2016, Hormel Food Corporation, a U.S-based meat food products company, launched a line of packaged ready-to-eat meals for cancer patients, which are called as Hormel Vital Cuisine.
These meals consist of carbohydrates, proteins, and fats to help patients fight loss of muscle mass and energy during cancer treatment. Thus launch…
Tumor Ablation Global Market From 2014 - 2024: Segmented Into Liver Cancer, Lung …
Researchmoz added Most up-to-date research on "Tumor Ablation Global Market From 2014 - 2024: Segmented Into Liver Cancer, Lung Cancer, Kidney Cancer, Bone Cancer, Breast Cancer, Prostate Cancer, And Others" to its huge collection of research reports.
Tumor ablation is the removal of the tumor cells or tissue with minimally invasive procedure. Tumor ablation devices are consists of an applicator (catheter), which is introduced into the tumor under imaging guidance. For…