Press release
AI-Based Clinical Trial Solution Providers Market Growth Drivers and Challenges Analysis Including Regulatory Dynamics and Adoption Barriers
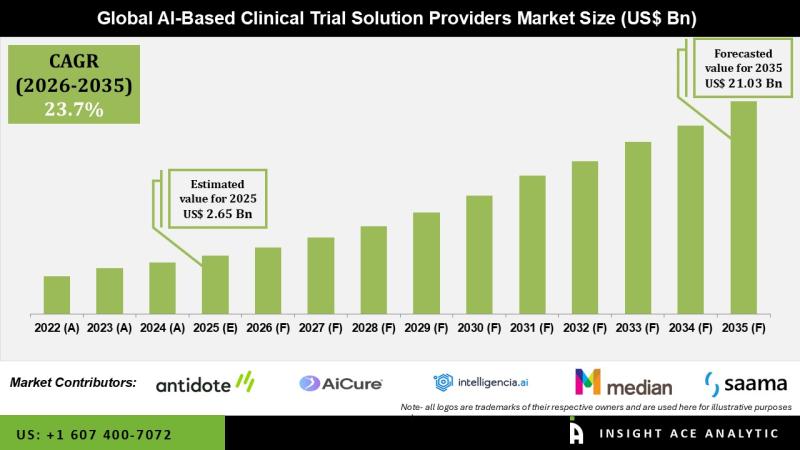
Global AI-Based Clinical Trial Solution Providers Market Size is valued at USD 2.65 Billion in 2025 and is predicted to reach USD 21.03 Billion by the year 2035 at a 23.7% CAGR during the forecast period for 2026 to 2035.Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/1082
According to the latest research by InsightAce Analytic, the global AI-based clinical trial solutions market is projected to achieve significant growth during the forecast period. Among regions, North America is expected to maintain a leading position in market share.
Artificial intelligence plays a pivotal role in clinical trial design and research, enabling the efficient analysis and interpretation of complex clinical data. AI-driven platforms allow researchers to simulate studies in silico, accelerating the evaluation of new molecules and therapies while addressing the intricate challenges of clinical research and medical product development.
Key factors supporting market growth include the rapid advancement of digital and medical technologies, continuous innovation in AI platforms and systems, the high prevalence of chronic and infectious diseases, increasing healthcare expenditures, growing government investment in clinical trial R&D, the expanding application of AI and machine learning techniques, and heightened awareness among researchers regarding AI capabilities. Additionally, biopharmaceutical companies are a major growth driver, as many increasingly leverage AI platforms and advanced trial design methodologies to expedite clinical development.
Nevertheless, stringent regulatory requirements governing AI-based clinical trial approvals and limited familiarity with AI applications among certain stakeholders may constrain market expansion over the forecast period (2020-2030).
From a regional perspective, North America dominated the market in 2020 and is expected to retain its leadership position, supported by a high incidence of health conditions, rapid adoption of innovative technologies, and increased government funding for clinical R&D. Europe is projected to follow, while the Asia-Pacific region is anticipated to experience substantial growth in the coming years, driven by a rising number of clinical trials and growing investments in research infrastructure.
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Major market players operating in the AI-based clinical trial solution providers market include Antidote Technologies, Inc. (US), AiCure, LLC (US), Deep 6 AI (US), Deep Lens Inc. (US), Innoplexus (Germany), Intelligencia.ai (US), MEDIAN Technologies (France), Mendel.ai (US), Phesi (US), Saama Technologies (US), Unlearn.AI, Inc. (US), and Trials.ai (US), among others.
A few of the developments in the market are as follows:
• In October 2021, Deep Lens (US) collaborated with Southern Oncology Specialists to use the AI-based solution VIPER for improving clinical trial matching. This partnership brings more precision medicine trials to the practice and broadens the practice's existing research program.
• In September 2021, Deep 6 AI (US), the leader in artificial intelligence (AI)-based clinical trial acceleration software (CTAS), introduced Deep 6 for Life Sciences, a novel suite of AI software solutions for life science sponsors and CROs, built on its industry-leading clinical trial acceleration platform. Deep 6 for Life Sciences brings a new level of data-driven precision and insight to sponsors' interventional and observational studies, saving them months and significantly de-risking their research efforts.
• In March 2020, Innoplexus AG (Germany) opened its proprietary Ontosight® AI search platform freely available to all medical researchers worldwide to help fight the COVID-19 pandemic.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customization/1082
Market Segments
Global AI-based Clinical Trial Solution Providers Market, by Target Therapeutic Area, 2020-2030 (Value US$ Mn)
• Cardiovascular Disorders
• CNS Disorders
• Infectious Disorders
• Metabolic Disorders
• Oncological Disorders
• Other Disorders
Global AI-based Clinical Trial Solution Providers Market, by Trial Phase, 2020-2030 (Value US$ Mn)
• Early Phase 1
• Phase 1
• Phase 2
• Phase 3
• Phase 4
Global AI-based Clinical Trial Solution Providers Market, by End-Users, 2020-2030 (Value US$ Mn)
• Pharmaceutical Companies
• Academia and Other Users
Global AI-based Clinical Trial Solution Providers Market, by Region, 2020-2030 (Value US$ Mn)
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East & Africa
North America AI-based Clinical Trial Solution Providers Market, by Country, 2020-2030 (Value US$ Mn)
• U.S.
• Canada
Europe AI-based Clinical Trial Solution Providers Market, by Country, 2020-2030 (Value US$ Mn)
• Germany
• France
• Italy
• Spain
• Russia
• Rest of Europe
Asia Pacific AI-based Clinical Trial Solution Providers Market, by Country, 2020-2030 (Value US$ Mn)
• India
• China
• Japan
• South Korea
• Australia & New Zealand
Latin America AI-based Clinical Trial Solution Providers Market, by Country, 2020-2030 (Value US$ Mn)
• Brazil
• Mexico
• Rest of Latin America
Middle East & Africa AI-based Clinical Trial Solution Providers Market, by Country, 2020-2030 (Value US$ Mn)
• GCC Countries
• South Africa
• Rest of Middle East & Africa
Read Overview Report- https://www.insightaceanalytic.com/report/global-ai-based-clinical-trial-solution-providers-market-/1082
Why should buy this report:
• To receive a complete analysis of the prospects for global AI-based clinical trial solution providers market
• To receive an industry overview and future trends of AI-based clinical trial solution providers market
• To analyze the AI-based clinical trial solution providers market drivers and challenges
• To get information on the AI-based clinical trial solution providers market value (US$ Mn) forecast to 2030
• Significant investments, mergers & acquisitions in the AI-based clinical trial solution providers market industry
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: https://www.insightaceanalytic.com/
Tel : +1 607 400-7072
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release AI-Based Clinical Trial Solution Providers Market Growth Drivers and Challenges Analysis Including Regulatory Dynamics and Adoption Barriers here
News-ID: 4356967 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…