Press release
Prostate Cancer Pipeline and Competitive Landscape: 150+ Companies, 160+ Therapies, and the Next Wave of FDA-Backed Innovation, analyses DelveInsight
Leading organizations including Curium, Merck, Telix Pharmaceuticals, Exelixis, AstraZeneca, AB Science, Lantheus, Pfizer, Jiangsu Hengrui Pharmaceuticals, Modra Pharmaceuticals, Bristol-Myers Squibb, Zenith Epigenetics, Xencor, Merus, Phosplatin Therapeutics, Laekna Therapeutics, Tavanta Therapeutics, Madison Vaccines, Taiho Pharmaceutical, Ipsen Biopharmaceuticals, LAVA Therapeutics, ESSA Pharma, Poseida Therapeutics, AbbVie, SL VAXiGEN, Nammi Therapeutics, BeiGene, DualityBio, and others.The prostate cancer treatment arena is undergoing rapid transformation, with over 150 organizations actively working on novel therapies across multiple stages of clinical development. Recent advancements include FDA fast-track recognitions, approvals of companion diagnostic tools, and positive clinical outcomes for established therapies such as NUBEQA Registered , Xtandi Trademark , and Cabometyx Registered . In parallel, late- and mid-stage investigational agents-including Janssen's Niraparib (Phase III), Zenith's ZEN-3694 (Phase II), Seagen's Ladiratuzumab Vedotin (Phase II), Fortis' FOR46 (Phase I/II), and Regeneron's REGN5678 (Phase I/II)-highlight a broad range of therapeutic strategies such as PARP inhibition, antibody-drug conjugates, and bispecific antibodies. Collectively, these developments underscore the strong innovation momentum and sustained investment focused on improving survival outcomes and quality of life for prostate cancer patients.
DelveInsight's "Prostate Cancer Pipeline Insight" delivers a comprehensive evaluation of the global prostate cancer pipeline, encompassing therapies at various clinical stages and showcasing the initiatives of leading pharmaceutical and biotechnology players. As per DelveInsight's analysis, more than 150 major companies are currently developing over 160 therapeutic candidates for prostate cancer. The report offers in-depth insights into clinical trial progress, mechanisms of action, administration routes, and recent developmental updates, while also assessing the long-term growth prospects of the prostate cancer treatment market.
Request your free sample of the Prostate Cancer Pipeline Report today to gain actionable intelligence, benchmark competitors, uncover opportunities, and optimize pipeline strategies @ [https://www.delveinsight.com/report-store/prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Key Highlights from the Prostate Cancer Pipeline Report
* DelveInsight's prostate cancer pipeline analysis reveals a highly dynamic ecosystem with over 150 active participants collectively advancing more than 160 pipeline therapies.
* Leading organizations-including Curium, Merck, Telix Pharmaceuticals, Exelixis, AstraZeneca, AB Science, Lantheus, Pfizer, Jiangsu Hengrui Pharmaceuticals, Modra Pharmaceuticals, Bristol-Myers Squibb, MacroGenics, Syntrix Pharmaceuticals, Zenith Epigenetics, Xencor, Merus, Phosplatin Therapeutics, Laekna Therapeutics, Tavanta Therapeutics, Madison Vaccines, Taiho Pharmaceutical, Kangpu Biopharmaceuticals, Arvinas, Candel Therapeutics, Blue Earth Therapeutics, Ipsen Biopharmaceuticals, LAVA Therapeutics, ESSA Pharma, Poseida Therapeutics, Janux Therapeutics, Aurigene Oncology, Sathgen Therapeutics, Full-Life Technologies, NextPoint Therapeutics, AbbVie, SL VAXiGEN, Sorrento Therapeutics, 858 Therapeutics, Avacta Life Sciences, Nammi Therapeutics, BeiGene, DualityBio, and others-are actively evaluating next-generation therapies to enhance prostate cancer care.
* A diverse set of promising pipeline assets-including 177Lu-PSMA-I&T, Opevesostat (MK-5684), 177Lu-DOTA-rosopatamab, Cabozantinib, Capivasertib, Masitinib, FPI-2265, 177Lu-PNT2002, Mevrometostat (PF-06821497), Fuzuloparib, ModraDoc006, BMS-986218, Lorigerlimab, SX-682, ZEN-3694, Vudalimab, OPDIVO (nivolumab), Zenocutuzumab, Vobramitamab Duocarmazine, PT-112, LAE201, TAVT-45, pTVG-HP (MVI-816), TAS-115, KPG-121, ARV-766, CAN-2409, Saruparib (AZD5305), 177Lu-rhPSMA-10.1, Tazemetostat, KEYTRUDA, LAVA-1207, Masofaniten (EPI-7386), P PSMA 101, JANX 007, AUR107, MSP008-22, 225Ac-FL-020, NPX267, ABBV-969, SL-T10, Abivertinib, ETX-19477, AVA 6000, QXL138AM, BG-68501, DB-1311, among others-are being assessed across different clinical phases.
* August 2025: Halda Therapeutics announced that the FDA granted Fast Track designation to its lead candidate HLD-0915 for the treatment of metastatic castration-resistant prostate cancer (mCRPC).
* July 2025: Trethera Corporation received FDA Fast Track designation for TRE-515, a first-in-class agent currently in Phase I trials, for use in combination with radioligand therapy for PSMA-positive mCRPC.
* July 2025: AB Science SA confirmed FDA and EMA authorization for a confirmatory Phase III trial of masitinib (study AB22007) in metastatic castration-resistant prostate cancer, targeting patients with less advanced metastatic disease using a biomarker-driven approach.
* June 2025: Bayer, in collaboration with Orion, announced FDA approval of darolutamide in combination with androgen deprivation therapy for metastatic castration-sensitive prostate cancer, supported by positive Phase III ARANOTE trial outcomes.
* May 2025: Candel Therapeutics received FDA RMAT designation for CAN-2409 in newly diagnosed localized prostate cancer patients at intermediate-to-high risk, in addition to its previously granted Fast Track status.
* March 2025: The FDA approved Telix Pharmaceuticals' NDA for TLX007-CDx (Gozellix), a diagnostic imaging agent for prostate cancer.
* March 2025: Quibim secured FDA 510(k) clearance for QP-Prostate Registered CAD, enhancing prostate lesion detection and diagnostic accuracy.
* February 2025: Ibex Medical Analytics obtained FDA 510(k) clearance for Ibex Prostate Detect, an AI-based pathology tool designed to identify subtle or rare prostate cancers.
* January 2025: The FDA granted Fast Track designation to Clarity Pharmaceuticals' 64Cu-SAR-bisPSMA PET imaging agent for detecting PSMA-positive lesions in patients with biochemical recurrence.
* September 2024: Ipsen reported that the Phase III CONTACT-02 trial of Cabometyx plus atezolizumab met its progression-free survival endpoint despite a non-significant overall survival improvement.
* August 2024: Nuvation Bio announced FDA clearance of its IND application for NUV-1511, the first clinical candidate from its proprietary drug-drug conjugate platform.
* July 2024: The FDA granted Fast Track designation to SYNC-T SV-102 for mCRPC.
* June 2024: Kangpu Biopharmaceuticals received FDA approval to initiate a Phase II/III trial of KPG-121 in combination with abiraterone for mCRPC.
* June 2024: BioNTech and Duality Biologics received FDA Fast Track designation for BNT324/DB-1311 in advanced or metastatic castration-resistant prostate cancer.
With more than 150 active developers and over 160 pipeline assets, the prostate cancer landscape is advancing at an unprecedented pace. Access DelveInsight's Prostate Cancer Pipeline Report to remain competitive @ [https://www.delveinsight.com/sample-request/prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Prostate Cancer Overview
Prostate cancer is among the most frequently diagnosed malignancies in men, particularly affecting those aged 50 and above. Originating in the prostate gland, which plays a key role in seminal fluid production, the disease can remain indolent for extended periods, although aggressive variants may metastasize rapidly to bones and other organs-making early diagnosis critical.
Major risk factors include aging, hereditary predisposition, genetic alterations, and lifestyle influences. Higher incidence rates are observed among African American men and individuals with a family history of prostate or breast cancer. Symptoms may involve urinary difficulties, reduced urine flow, pelvic pain, or the presence of blood in urine, though early-stage disease often remains asymptomatic.
Diagnosis typically relies on PSA testing, digital rectal examination, imaging modalities such as MRI, and confirmatory biopsy. Treatment approaches vary based on disease stage and patient health, ranging from active surveillance and surgery to radiation, hormonal therapy, chemotherapy, and emerging targeted or immunotherapies.
Improved disease awareness, technological advances in diagnostics, and innovative treatments continue to enhance survival rates and patient outcomes. Early screening and personalized care remain central to effective prostate cancer management.
Download free sample report here @ Prostate Cancer Companies and FDA Approvals [https://www.delveinsight.com/sample-request/prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Snapshot of Selected Pipeline Therapies
* 177Lu-PSMA-I&T - Curium
* Opevesostat (MK-5684; ODM-208) - Merck / Orion
* Mevrometostat (PF-06821497) - Pfizer
* TRUQAP (capivasertib) - AstraZeneca
* 177Lu-PNT2002 - Lantheus
* 177Lu-DOTA-rosopatamab (TLX591) - Telix Pharmaceuticals
* TAVT-45 - Tavanta Therapeutics
* Saruparib (AZD5305) - AstraZeneca
* CAN-2409 - Candel Therapeutics
* Fuzuloparib - Jiangsu Hengrui Pharmaceuticals
Explore breakthrough therapies, regulatory developments, and late-stage trials shaping the future of prostate cancer care @ Prostate Cancer Companies and FDA Approvals [https://www.delveinsight.com/sample-request/prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Bottom of Form
Scope of the Prostate Cancer Pipeline Report
* Coverage: Global
* Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
* Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
* Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
* Therapeutics Assessment By Molecule Type: Monoclonal antibody, Peptides, Polymer, Small molecule, Gene therapy
* Therapeutics Assessment By Mechanism of Action: PSMA inhibitors (Prostate-specific Membrane Antigen Inhibitors), CYP11A1 inhibitor, EZH2 inhibitor, Proto-oncogene protein c-akt inhibitor, PSMA-targeted therapy, Ionising radiation emitter, Steroidal inhibitor of CYP17A1, Poly(ADP-ribose) polymerase-1 inhibitor, Thymidine kinase expression stimulants, Poly(ADP-ribose) polymerase 2 inhibitors
* Key Prostate Cancer Companies: Merck & Co., Inc. (NYSE: MRK), Telix Pharmaceuticals Limited (ASX: TLX), Exelixis, Inc. (NASDAQ: EXEL), AstraZeneca PLC (NASDAQ: AZN), AB Science S.A. (EPA: AB), Lantheus Holdings, Inc. (NASDAQ: LNTH), Pfizer Inc. (NYSE: PFE), Bristol-Myers Squibb Company (NYSE: BMY), MacroGenics, Inc. (NASDAQ: MGNX), Xencor, Inc. (NASDAQ: XNCR), Merus N.V. (NASDAQ: MRUS), Arvinas, Inc. (NASDAQ: ARVN), Candel Therapeutics, Inc. (NASDAQ: CADL), Ipsen S.A. (EPA: IPN), LAVA Therapeutics N.V. (NASDAQ: LVTX), ESSA Pharma Inc. (NASDAQ: EPIX), Poseida Therapeutics, Inc. (NASDAQ: PSTX), Janux Therapeutics, Inc. (NASDAQ: JANX), AbbVie Inc. (NYSE: ABBV), Sorrento Therapeutics, Inc. (OTC: SRNEQ), Avacta Group plc (LSE: AVCT), BeiGene, Ltd. (NASDAQ: BGNE), Duality Biologics (HKEX: 6988), Curium, Modra Pharmaceuticals, Syntrix Pharmaceuticals, Zenith Epigenetics, Phosplatin Therapeutics, Laekna Therapeutics, Tavanta Therapeutics, Madison Vaccines, Taiho Pharmaceutical, Kangpu Biopharmaceuticals, Blue Earth Therapeutics, Aurigene Oncology, Sathgen Therapeutics, Full-Life Technologies, NextPoint Therapeutics, SL VAXiGEN, Nammi Therapeutics, and 858 Therapeutics, and others
* Key Prostate Cancer Pipeline Therapies: 177Lu-PSMA-I&T, Opevesostat (MK-5684), 177Lu-DOTA-rosopatamab, Cabozantinib, Capivasertib, Masitinib, FPI-2265, 177Lu-PNT2002, Mevrometostat (PF-06821497), Fuzuloparib, ModraDoc006, BMS-986218, Lorigerlimab, SX-682, ZEN-3694, Vudalimab, OPDIVO (nivolumab), Zenocutuzumab, Vobramitamab Duocarmazine, PT-112, LAE201, TAVT-45, pTVG-HP (MVI-816), TAS-115, KPG-121, ARV-766, CAN-2409, Saruparib (AZD5305), 177Lu-rhPSMA-10.1, Tazemetostat (Tazverik), KEYTRUDA, LAVA-1207, Masofaniten (EPI-7386), P PSMA 101, JANX 007, AUR107, MSP008-22, 225Ac-FL-020, NPX267, ABBV-969, SL-T10, Abivertinib, ETX-19477, AVA 6000, QXL138AM, BG-68501, DB-1311, and others
Explore the latest prostate cancer pipeline therapies - from PARP inhibitors to next-gen ADCs and bispecifics. Get competitive insights on Janssen, GSK, Seagen, Fortis & Regeneron. @ [https://www.delveinsight.com/sample-request/prostate-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=prostate-cancer-pipeline-and-competitive-landscape-150-companies-160-therapies-and-the-next-wave-of-fdabacked-innovation-analyses-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Prostate Cancer Pipeline and Competitive Landscape: 150+ Companies, 160+ Therapies, and the Next Wave of FDA-Backed Innovation, analyses DelveInsight here
News-ID: 4352095 • Views: …
More Releases from ABNewswire
Neuroblastoma Market Size and Forecast (2025-2034): 7 Major Markets, 700-800 US …
Major players active in the neuroblastoma treatment space include United Therapeutics, EUSA Pharma, Y-mAbs Therapeutics, Clarity Pharmaceuticals, along with several other companies.
The Neuroblastoma Treatment Market is expected to witness substantial growth through 2034. Nearly 90% of neuroblastoma cases are diagnosed in children younger than five years, and close to half of high-risk patients experience disease relapse after achieving initial remission, while around 15% develop refractory disease. Standard treatment approaches comprise…
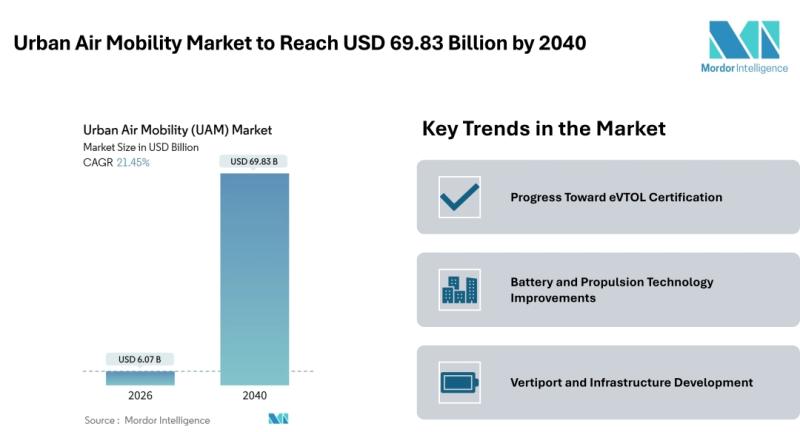
Urban Air Mobility Market to Reach USD 69.83 Billion by 2040, Driven by eVTOL Co …
Mordor Intelligence has published a new report on the Urban air mobility market offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
The Urban Air Mobility Market size [https://www.mordorintelligence.com/industry-reports/urban-air-mobility-uam-market?utm_source=abnewswire] is poised for transformational expansion, growing from an estimated USD 6.07 billion in 2026 to USD 69.83 billion by 2040, registering a CAGR of 21.45% during the forecast period. This growth is driven by rapid advancements in electric vertical takeoff…

5G Services Market Global Growth, Key Opportunities, Latest Trends, Top Companie …
5G Services Market Size, Share, Growth Analysis, by Communication Type, End User (Consumers and Enterprises), Application (Industry 4.0, Smart Cities), Enterprise (Manufacturing, Transportation & Logistics), and Region - Global Forecast to 2028.
The 5G services market [https://www.marketsandmarkets.com/Market-Reports/5g-services-market-226908556.html?utm_campaign=5gservicesmarket&utm_source=abnewswire.com&utm_medium=referral] is expected to expand at a compound annual growth rate (CAGR) of 19.3%, from USD 205.52 billion in 2023 to USD 497.24 billion by 2028. Transformative technologies like virtual reality (VR), augmented reality (AR),…

Software Asset Management Market Growing Trends, Top Business Strategy, Healthy …
Software Asset Management Market by Solutions (License Management, Audit & Compliance Management, Software Discovery, Optimization, & Metering, Contract Management, Configuration Management) - Global Forecast to 2029
The software asset management market [https://www.marketsandmarkets.com/Market-Reports/software-asset-management-market-235932482.html?utm_campaign=softwareassetmanagementmarket&utm_source=abnewswire.com&utm_medium=referral] is projected to expand from USD 3.5 billion in 2024 to USD 7.3 billion by 2029 at a Compound Annual Growth Rate (CAGR) of 16.0% over the course of the forecast period. The move to cloud computing, expanding regulatory…
More Releases for Therapeutic
Exosome Therapeutic Market : Detailed Overview
Introduction:
The exosome therapeutic market is a rapidly emerging segment in the field of regenerative medicine and drug delivery. Exosomes are extracellular vesicles naturally released by cells that play a crucial role in cell communication and genetic material transfer. They have gained attention for their potential applications in treating various diseases, including cancer, neurodegenerative disorders, and cardiovascular conditions. As a novel therapeutic platform, exosomes provide significant advantages such as low immunogenicity,…
Parkinson's Disease Therapeutic Market Hope for Patients: The Parkinson's Diseas …
Parkinson's Disease Therapeutic Market worth $6.51 Bn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Parkinson's Disease Therapeutic Market - (By Drugs (Carbidopa-levodopa, Dopamine agonists, Mao-b inhibitors, COMT inhibitors, Anticholinergics, Others), By Distribution Channel (Hospital pharmacy, Retail pharmacy, Online pharmacy), By Brand (Branded, Generics), By Route of Administration (Oral, Injectable, Intestinal Infusion, Subcutaneous, Others)),…
Global Remote Therapeutic Monitoring Market
According to a new market research report published by Global Market Estimates, the Global Remote Therapeutic Monitoring Market is projected to grow at a CAGR of 17.4% from 2023 to 2028.
Medsien, Intellihinc, Zimmer Biomet, Owlytics Healthcare, Limber Health, Medistics, RxCap, Propeller Health, HealthArc, CENSON Health, and B Castle Smith & Co., are some of the key players in the remote therapeutic monitoring market.
Browse 147 Market Data Tables and 115…
Europe Digital Therapeutic (DTx) Market
Europe Digital Therapeutic (DTx) Market report provides information about the industry, including valuable Analysis and Detailed study. This research study explores the Global Europe Digital Therapeutic (DTx) Market in detail such as industry chain structures, raw material suppliers, with manufacturing. The Europe Digital Therapeutic (DTx) market examines the primary segments of the market. This intelligent study provides historical data from forecast.It also provides the details such as whether the customers…
Recombinant Therapeutic Antibodies and Proteins Market continues to expand with …
Recombinant therapeutic protein drugs are an important class of medicines, which helps patients in need of novel therapies. Recently approved recombinant protein therapeutics have been developed to treat a wide variety of clinical indications, including cancers, inflammation/autoimmunity, genetic disorders, and exposure to infectious agents. The latest advancements in protein-engineering technologies have allowed drug manufacturers and developers to adjust desirable functional characteristics of proteins of interest maintaining product efficacy. Protein-based therapies…
Therapeutic Vaccine Market 2020 - 2028 Top Companies Agenus Inc., Argos Therapeu …
This detailed market study covers therapeutic vaccine market growth potentials which can assist the stake holders to understand key trends and prospects in therapeutic vaccine market identifying the growth opportunities and competitive scenarios. The report also focuses on data from different primary and secondary sources, and is analyzed using various tools. It helps to gain insights into the market's growth potential, which can help investors identify scope and opportunities. The…