Press release
Fabry Disease Market Valued at USD 1.7 Billion in 2024, According to DelveInsight
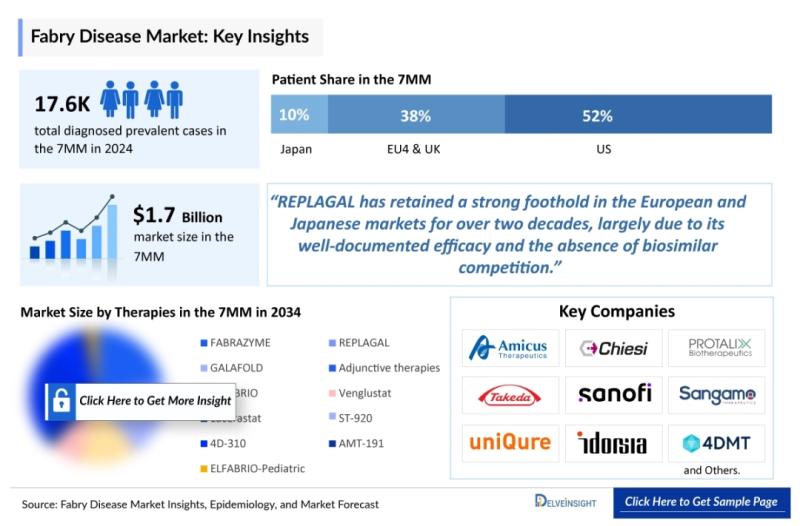
Key companies active in the Fabry Disease space include Protalix Biotherapeutics, Sanofi Genzyme, Sangamo Therapeutics, Freeline Therapeutics, 4D Molecular Therapeutics, Idorsia Pharmaceuticals, GREENOVATION BIOTECH GMBH, Shire, Takeda, Amicus Therapeutics, Protalix, uniQure, Codexis, MP6 Therapeutics, and CellGenTech.DelveInsight estimates that the Fabry Disease market reached a value of USD 1.7 billion in 2024. In the same year, the United States led the Fabry Disease therapeutics market, accounting for approximately 52% of the total 7MM market and generating close to USD 880 million-the largest share across all regions. The market is anticipated to witness robust growth through 2034. Fabry disease is a rare X-linked genetic disorder caused by mutations in the alpha-galactosidase A gene, resulting in progressive damage to multiple organs. While advances in newborn screening programs are enabling earlier diagnosis, a significant proportion of adults continue to experience severe disease-related complications by mid-life.
Currently available treatment options for Fabry disease include enzyme replacement therapies (ERTs) and pharmacological chaperone therapy. Prominent marketed therapies-FABRAZYME, REPLAGAL, GALAFOLD, and ELFABRIO-utilize different mechanisms to address the underlying enzyme deficiency. In the US, FABRAZYME remains the most widely used ERT, whereas REPLAGAL has a strong foothold in Europe and Japan. ELFABRIO, the most recent market entrant, has received approval in both the US and EU, with ongoing regulatory efforts aimed at enabling a monthly dosing schedule.
The competitive landscape comprises leading pharmaceutical and biotechnology companies such as Amicus Therapeutics, Sanofi (Genzyme), Takeda, CHIESI-Protalix, Sangamo Therapeutics, and uniQure. The development pipeline includes several promising assets, including Venglustat, the gene therapy candidate ST-920, and additional therapies such as lucerastat, 4D-310, and AMT-191-although some candidates have encountered efficacy or safety-related challenges. Other early-stage therapies, including AceLink's AL01211, are also demonstrating initial promise.
Continued progress in clinical research, rapid innovation in gene therapies, increasing disease awareness, and improvements in diagnostic capabilities are expected to fuel future market expansion and enhance patient outcomes in Fabry disease.
DelveInsight's report, "Fabry Disease Market Insights, Epidemiology, and Market Forecast - 2034," provides a comprehensive overview of Fabry disease, covering historical and projected epidemiology as well as market trends across the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan. The report analyzes current treatment standards, emerging therapies, individual therapy market shares, and both historical and forecasted market size from 2020 to 2034 across the seven major markets. Additionally, it evaluates existing treatment algorithms, key market drivers, barriers, and unmet needs to identify growth opportunities and assess the overall potential of the Fabry Disease market.
Request for a Free Sample Report @ [https://www.delveinsight.com/report-store/neuropathic-pain-market?utm_source=benzinga&utm_medium=pressrelease&utm_campaign=apr]
Key Highlights from the Fabry Disease Market Report
* According to DelveInsight, the Fabry Disease market is projected to expand at a steady CAGR through 2034.
* DelveInsight analysis indicates that the total Fabry Disease market size across the 7MM was approximately USD 1.7 billion in 2024.
* In 2022, the diagnosed prevalent population of Fabry disease across the 7MM stood at around 15,290 cases, with numbers expected to rise throughout the forecast period (2019-2034).
* Major companies operating in the Fabry Disease market include Protalix Biotherapeutics, Sanofi Genzyme, Sangamo Therapeutics, Freeline Therapeutics, 4D Molecular Therapeutics, Idorsia Pharmaceuticals, GREENOVATION BIOTECH GMBH, Shire, Takeda, Amicus Therapeutics, uniQure, Codexis, MP6 Therapeutics, CellGenTech, and others.
* Key therapies anticipated to enter the market include PRX-102 (Pegunigalsidase alfa), Venglustat, ST-920, FLT190, 4D-310, Lucerastat, Moss-aGal, among others.
* May 2025: All patients treated with Sangamo Therapeutics' gene therapy ST-920 (isaralgagene civaparvovec) in the Phase 1/2 trial reached the FDA-mandated one-year follow-up milestone, enabling progression toward accelerated approval. Sangamo expects pivotal data by the end of June, and the FDA has previously indicated that Phase 1/2 STAAR trial data may be sufficient for accelerated approval, potentially bypassing additional trials.
* March 2025: Sangamo Therapeutics confirmed alignment with the FDA on an accelerated approval pathway for ST-920, with plans to submit a BLA in the second half of 2025.
* February 2025: Amicus Therapeutics showcased oral presentations and posters on its migalastat development programs at the 21st Annual WORLD Symposium.
* January 2025: Idorsia Pharmaceuticals announced expectations for Phase III Open-Label Extension study results in Q2 2025, followed by discussions with the US FDA on the regulatory pathway.
* January 2025: Sanofi disclosed that regulatory submission for venglustat in Fabry disease is targeted for 2026.
* December 2024: CHIESI Farmaceutici and Protalix BioTherapeutics reported EMA validation of a variation application for pegunigalsidase alfa, supporting a less frequent dosing regimen of 2 mg/kg every four weeks in adult Fabry patients.
* September 2024: uniQure stated that its Phase I/IIa gene therapy program is expected to generate initial proof-of-concept data in 2025.
* March 2024: Sanofi released Phase IV study results assessing the safety and tolerability of Fabrazyme at higher infusion rates and reduced volumes.
* April 2024: 4D Molecular Therapeutics announced results from an open-label Phase 1/2a study of gene therapy 4D-310 in adults with Fabry disease and cardiac involvement.
Fabry Disease Overview
Fabry disease is a rare X-linked lysosomal storage disorder caused by pathogenic variants in the GLA gene, leading to reduced activity of the -galactosidase A enzyme. This deficiency results in the accumulation of globotriaosylceramide (Gb3) in multiple organs, particularly the kidneys, heart, and nervous system. Clinical manifestations range from neuropathic pain, heat intolerance, angiokeratomas, and gastrointestinal symptoms to severe complications such as renal failure, cardiomyopathy, arrhythmias, and stroke. Although both males and females are affected, disease severity varies, with late-onset forms being more prevalent and often diagnosed during adulthood. Expanded newborn screening and genetic testing are improving early detection. Current treatments include ERTs such as FABRAZYME and REPLAGAL, as well as the oral chaperone therapy GALAFOLD, all aimed at reducing substrate accumulation and slowing disease progression. Emerging therapies, including substrate reduction approaches and gene therapies, offer the potential for more durable and targeted disease control.
Learn more about Fabry Disease treatment algorithms in different geographies, and patient journeys. Contact to receive a sample @ [https://www.delveinsight.com/sample-request/fabry-disease-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Fabry Disease Market Outlook
The Fabry Disease therapeutics market across the 7MM was valued at approximately USD 1.7 billion in 2024, with the United States accounting for nearly 52% (around USD 880 million) of total revenue. Europe followed, led by Germany (approximately USD 210 million), while Spain represented the smallest share (around USD 82 million). Japan contributed nearly USD 150 million, or about 9% of the total market.
Fabry disease arises from -Gal A enzyme deficiency, leading to progressive organ damage. Available treatments include ERTs-FABRAZYME, REPLAGAL, and ELFABRIO-and the oral chaperone therapy GALAFOLD, which is effective in roughly 35-50% of patients with amenable GLA mutations. While ERTs remain the backbone of therapy, challenges such as frequent intravenous infusions and the development of antidrug antibodies persist.
Emerging therapies, including venglustat, ST-920, 4D-310, and AMT-191, aim to enhance enzyme delivery and introduce gene-based treatment options. Market growth is being driven by rising prevalence, improved diagnostic rates, and pipeline innovation, although pediatric treatment gaps and long-term durability remain key concerns.
Fabry Disease Market Drivers
* Growing Awareness and Early Diagnosis: Expanded newborn screening and greater awareness are enabling earlier identification and treatment.
* Limited Approved Therapies: A small number of existing treatment options creates strong demand for more effective alternatives.
* Pipeline Advancement: Innovation in next-generation ERTs, SRTs, and gene therapies is broadening therapeutic possibilities.
* Regulatory Progress: Approvals and label expansions for therapies such as ELFABRIO and GALAFOLD support market expansion.
* High Unmet Medical Need: Ongoing organ damage and disease complications drive demand for therapies that improve survival and quality of life.
Fabry Disease Market Barriers
* High Cost of Treatment: Expensive therapies pose access and reimbursement challenges.
* Complex Clinical Management: Phenotypic variability and late-onset disease complicate treatment decisions.
* Lack of Curative Options: Current treatments are disease-modifying rather than curative; gene therapies remain investigational.
* Clinical Development Risks: Some pipeline assets have faced setbacks related to safety or efficacy.
* Small Patient Population: Limited prevalence can restrict commercial scalability and R&D investment.
Fabry Disease Epidemiology
In 2024, the United States accounted for approximately 9,200 diagnosed prevalent Fabry disease cases, representing nearly 52% of the total 7MM population. The EU4 and the UK together comprised about 38%, while Japan contributed roughly 10%. Germany reported the highest number of diagnosed cases in Europe (~2,170), whereas Spain had the lowest.
In the US, late-onset Fabry disease predominated, with around 5,860 cases, compared to approximately 3,300 classic cases. By age group, prevalence peaked among individuals aged 10-19 years (~1,920 cases), followed by 20-29 years (~1,780), 30-39 years (~1,700), and 40-49 years (~1,430), with about 1,180 cases each in those under 10 years and over 50 years. These patterns highlight the dominance of late-onset disease and underscore the importance of early diagnosis and timely intervention.
Fabry Disease Epidemiology Segmentation
* Total diagnosed prevalent cases
* Gender-specific diagnosed prevalent cases
* Age-specific diagnosed prevalent cases
* Phenotype-specific diagnosed prevalent cases
Explore more about Fabry Disease Epidemiology @ [https://www.delveinsight.com/report-store/fabry-disease-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
According to DelveInsight, Fabry disease prevalence is higher in males than females across the 7MM, although regional variations exist.
Fabry Disease Competitive Landscape (Paraphrased)
* Venglustat (Sanofi/Genzyme): An oral substrate reduction therapy designed to inhibit glucosylceramide synthase, reducing glycosphingolipid accumulation. Late-stage development is ongoing, with Phase III data expected in H2 2025 and potential regulatory submissions in 2026.
* Lucerastat (Idorsia): A small-molecule SRT applicable across Fabry phenotypes. Although the Phase III MODIFY trial missed its primary endpoint, long-term OLE data expected in Q2 2025 may support its future role. The therapy holds orphan designation in the US and EU.
* ST-920 (Sangamo Therapeutics): A liver-targeted AAV2/6 gene therapy enabling sustained -Gal A production following a single IV dose. Encouraging safety and durability data support ongoing accelerated approval discussions, with a BLA planned for H2 2025.
* AMT-191 (uniQure): An AAV5-based gene therapy designed for long-term correction of -Gal A deficiency. Early-stage trials are progressing, with FDA orphan and fast-track designations supporting expedited development.
Request for a sample report to understand more about the Fabry Disease pipeline development activities @ [https://www.delveinsight.com/sample-request/fabry-disease-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Fabry Disease Pipeline Therapies & Key Companies
* PRX-102 (Pegunigalsidase alfa): Protalix Biotherapeutics
* Venglustat: Sanofi Genzyme
* ST-920: Sangamo Therapeutics
* FLT190: Freeline Therapeutics
* 4D-310: 4D Molecular Therapeutics
* Lucerastat: Idorsia Pharmaceuticals
* Moss-aGal: GREENOVATION BIOTECH GMBH
Fabry Disease Therapeutics Assessment
Numerous companies are actively advancing innovative therapies in the Fabry Disease market, including Protalix Biotherapeutics (NYSE: PLX), Sanofi Genzyme (EPA: SAN), Sangamo Therapeutics (NASDAQ: SGMO), Freeline Therapeutics (NASDAQ: FRLN), 4D Molecular Therapeutics (NASDAQ: FDMT), Idorsia Pharmaceuticals (SWX: IDIA), GREENOVATION BIOTECH GMBH, Shire (formerly NASDAQ: SHPG), Takeda (TSE: 4502), Amicus Therapeutics (NASDAQ: FOLD), uniQure (NASDAQ: QURE), Codexis (NASDAQ: CDXS), MP6 Therapeutics, CellGenTech, Protalix (NYSE: PLX) and others.
Learn more about the emerging Fabry Disease therapies & key companies @ [https://www.delveinsight.com/sample-request/fabry-disease-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Fabry Disease Report Key Insights
* Patient population analysis
* Market size and growth trends
* Competitive dynamics
* Market drivers and barriers
* Emerging opportunities
* Therapeutic strategies
* Pipeline evaluation
* Current treatment algorithms
* Impact of emerging therapies
About DelveInsight
DelveInsight is a premier life sciences market research and business consulting firm, widely recognized for its syndicated research reports and customized solutions tailored to the healthcare industry.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=fabry-disease-market-valued-at-usd-17-billion-in-2024-according-to-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting/conference-coverage-services
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Fabry Disease Market Valued at USD 1.7 Billion in 2024, According to DelveInsight here
News-ID: 4351921 • Views: …
More Releases from ABNewswire

Love Pines Realty Announces New Home for Sale in Moore County
146 Union Church Road, Vass, North Carolina
Vass, North Carolina (February 26, 2026) - Love Pines Realty is proud to announce its newest home for sale at 146 Union Church Road in Vass, NC, offering one of the best values in today's competitive Moore County real estate market.
Image: https://www.abnewswire.com/upload/2026/02/6d5716a3a4fa4bf07ff3e915e3d07b89.jpg
Priced at $365,000, this charming ranch-style home is ideal for buyers searching for a home for sale in Vass, NC with modern updates,…

Best Luxury Villas in Greece for Families, Couples & Groups
When privacy is more important than room service, and space is more important than square footage, villa vacations become the obvious choice. Today's luxury travelers do more than just reserve their accommodations. They are deciding how they want to live on their vacation. A well-chosen villa offers something hotels rarely do: personal space without compromise. And a setting that is comfortable for families, couples, and groups alike.
The following are three…

Lazar Law Firm Launches Newly Redesigned Website to Enhance Client Experience Ac …
Lazar Law Firm is proud to announce the launch of its newly redesigned website, created to
London, Ontario - Lazar Law Firm is proud to announce the launch of its newly redesigned website, created to deliver a more streamlined, informative, and client-focused online experience.
The updated website reflects the firm's continued commitment to professionalism, clarity, and accessibility. With improved navigation, expanded service pages, and a fully responsive design, clients can now more…

Best SMP in New York - What 4-Hour Sessions Really Mean
See what 4-hour SMP sessions in New York truly mean, why proper time ensures natural results, and how Masters delivers lasting hairlines.
Image: https://www.abnewswire.com/upload/2026/02/8767eb21516e48c2b026fbd2a133316f.jpg
Have you ever looked in the mirror and barely recognized yourself? Not because of age, but because your hairline does not match the person you remember being. That moment hits hard for anyone dealing with hair loss. The decision to do something about it brings real anxiety. Then…
More Releases for Fabry
Key Factor Supporting Global Fabry Disease Treatment Market Development in 2025: …
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Fabry Disease Treatment Market Size By 2025?
The valuation of the Fabry disease treatment market has experienced robust expansion recently, projected to advance from $2.09 billion in 2024 up to $2.27 billion by 2025, reflecting an 8.8% compound annual growth rate during this timeframe. This…
U.S. Fabry Disease Market Size Report 2034
On April 28, 2025, Exactitude Consultancy., Ltd. released a research report titled "U.S. Fabry Disease Market". This report covers the global U.S. Fabry Disease market sales, sales volume, price, market share, ranking of major companies, etc., and provides a detailed analysis by region, country, product type, and application. It also forecasts the market size of automotive kick sensors based on market patterns from 2020 to 2034 and future market trends.…
Top Factor Driving Fabry Disease Treatment Market Growth in 2025: Impact of Incr …
How Are the key drivers contributing to the expansion of the fabry disease treatment market?
The rising prevalence of renal diseases is expected to drive the growth of the Fabry disease treatment market. Renal diseases are becoming more prevalent due to genetic factors, lifestyle choices, and environmental influences. Fabry disease, which causes kidney dysfunction, is increasing and requires timely intervention. According to the Australian Bureau of Statistics, kidney disease affected 246,200…
Fabry Disease Market Trends Analysis 2030
Fabry disease is a rare X-linked lysosomal storage disorder. This patient has a deficiency in the enzyme alpha galactosidase, which progresses to organ failure. The development of fabry illnesses is mostly caused by abnormal accumulation of a certain fatty substance known as globotriaosylceramide. This aberrant buildup can be detected in the skin, eyes, heart, kidney, brain, gastrointestinal system, and central nervous system, among other body parts.
Galactosidase Alpha (GLA) is a…
Fabry Disease - Pipeline Review, H1 2017
ReportsWorldwide has announced the addition of a new report title Fabry Disease - Pipeline Review, H1 2017 to its growing collection of premium market research reports.
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Fabry Disease - Pipeline Review, H1 2017, provides an overview of the Fabry Disease (Genetic Disorders) pipeline landscape.
Fabry disease is an inherited disorder. Fabry disease results from abnormal deposits of a particular fatty substance (called…
Fabry Disease Market Intelligence Report Offers Growth Prospects
Fabry diseaseis also known as Anderson-Fabry disease and alpha-galactosidase A deficiency. It is a rare genetic disorder of lipid metabolism resulting from the deficient activity of the alpha-galactosidase A (a-Gal A) enzyme. The deficiency of the enzyme is caused by the alterations in the genes that instructs the cells to make alpha-galactosidase A (a-Gal A) enzyme. Fabry disease is known to cause variety of systemic symptoms and complications, one of…