Press release
How CE/MDR Compliant Breathing Improvement Devices Factory Meets Rising Wellness Needs
As healthcare trends increasingly move toward noninvasive, home-based therapies, the need for high-standard medical technologies has skyrocketed. Careboo has emerged as an industry leader in non-invasive sleep and respiratory health, positioning itself as a premier CE/MDR compliant breathing improvement devices(https://www.careboohealth.com/product-category/snoring/) factory that is redefining the standards of wearable therapy. By applying cutting-edge materials science combined with stringent regulatory adherence, the company addresses the root physiological causes of sleep disorders, helping millions regain high-quality rest through advanced monitoring and stimulation technology.Industry Outlook: Home-Based Wellness
The global market for sleep health and respiratory therapy is currently experiencing a monumental transformation, due to a rising aging population as well as increasing awareness of cardiovascular risks associated with untreated snoring and sleep apnea, driving this industry toward physical and technological interventions rather than pharmaceutical dependencies. Market data projects a compound annual compound rate growth projection of over 7% through 2030 with portable, non-invasive solutions seeing particular surges in demand.
Modern consumers no longer settle for simple mechanical fixes; they require "smart" healthcare that integrates monitoring, testing and treating devices into one device. Thanks to the "Retailization of Healthcare," devices that were once reserved for sleep labs have now become household wellness tools - creating a monumental opportunity for CE/MDR compliant breathing improvement devices factories to provide crucial recovery and respiratory efficiency as foundational elements of vitality in today's fitness-driven "Silver Economy."
Trust Standard: Global Certifications and Exhibition Excellence
In medical exporting, trust must be built through extensive testing. Careboo's dedication to quality is demonstrated by its impressive collection of international certifications; Careboo has successfully made the transition to European Union Medical Device Regulation (MDR), receiving CE marking and ISO 13485 quality management system certification - in addition to FDA 510(k) clearance for North American markets.
Careboo has earned itself a trusted place on the global exhibition circuit by virtue of these credentials, appearing regularly at some of the world's premier medical trade fairs such as:
Arab Health (Dubai): Arab Health is the Middle East's premier healthcare event.
MEDICA (Dusseldorf): MEDICA is the world's premier medical trade fair.
Hospitalar (So Paulo): the gateway to Latin American healthcare market.
FIME (Miami): FIME is the central hub for North and South American medical trade.
Careboo showcases its CE/MDR compliant factory at events around the globe, showing how its high-precision tools comply with legal and clinical requirements, giving distributors confidence in introducing these technologies into different markets around the globe.
As a CE/MDR compliant breathing improvement devices factory, Careboo addresses the rising wellness demands related to respiratory and sleep disorders through non-invasive interventions that use electrical pulses, pressure, heat, cold and light technology - an approach known as multiphysics wellness.
Breakthroughs in Snoring and Respiratory Health
Careboo has achieved remarkable progress in treating jaw muscle and respiratory soft tissue weakness. By applying precise electrical pulses, Careboo's anti-snoring devices stimulate and tone airway muscles preventing their collapse which causes snoring. This makes revolutionary progress towards stopping or reducing snoring while providing an elegant alternative to invasive surgeries or loud CPAP machines.
Careboo's product ecosystem was designed to address pain relief, vitality enhancement and relaxation needs of individuals dealing with:
Physical injuries and joint strain: Leveraging TENS/EMS technology for fast recovery.
Exercise and fitness: Aiding muscle recovery and increasing vitality.
Sleep quality testing: Offering advanced monitoring to give users an exceptional experience of care.
Careboo products use only high-grade materials and follow ergonomic design concepts, to ensure our users receive optimal care from modern technology. From Sleep Therapy Monitors and TENS Units to Red Light Therapy devices, our customers benefit from care provided by Careboo. Not just manufacturers; Careboo is part of a global journey toward better sleep and an enriching life.
Careboo has long been dedicated to the research, development and technology for various sleep and pain disorders. By blending CE/MDR compliance with an empathic design philosophy that puts consumers first, Careboo has set new expectations of home-care medical devices.
For more information about our breathing improvement solutions, product catalogs or global partnership inquiries, please visit our official website.
Media Inquires should contact Careboo Health's Marketing Department or visit https://careboohealth.com/ for more information.
ROOM A, 3/F., WING TAT COMMERCIAL BUILDING, 121-125 WING LOK STREET, SHEUNG WAN HK
footer_icon_2info@careboohealth.com
Careboo specializes in sleep health and healthcare products, with long-standing dedication to R&D and technological innovation for sleep and pain disorders. Boasting remarkable achievements in sleep quality testing and improvement, we deliver premium sleep experiences via advanced monitoring technology.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release How CE/MDR Compliant Breathing Improvement Devices Factory Meets Rising Wellness Needs here
News-ID: 4348591 • Views: …
More Releases from CAREBOO CO., LIMITED

China CAREBOO Respiratory Device Manufactor CE/FDA Highlights Compliance Strengt …
Careboo, a China CE/FDA respiratory device manufacturer(https://www.careboohealth.com/product-category/snoring/), is delighted to join Arab Health 2026 at Dubai Exhibition Centre as an exhibitor and take advantage of this premier platform to demonstrate their latest advancements in sleep health and respiratory therapy, emphasizing their rigorous commitment to international safety standards and therapeutic efficacy for an international audience.
The Global Sleep Health Horizon: Industry Prospects and Trends
The global market for sleep apnea and respiratory devices…
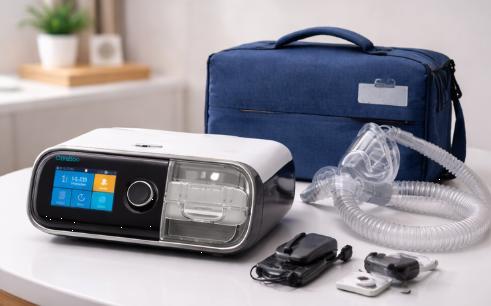
CAREBOO Air Breathing Equipment Manufacturer China Introduces New Respiratory Sy …
As global respiratory awareness and the prevalence of sleep-related breathing disorders increase, so has the demand for advanced, safe, and patient-centric respiratory systems. CAREBOO air breathing equipment manufacturer China is emerging as a leader in this space-offering high-quality solutions across hospitals, sleep clinics, and homecare settings. Its innovative range combines advanced technology, rigorous regulatory compliance, and patient-centric design, setting benchmarks in respiratory therapy while unveiling its latest innovations at the…

MDR Compliant Breathing Device Manufactor China vs Low-Cost Alternatives
Global healthcare standards have reached a crucial turning point, prompting an unprecedented need for high-performance respiratory therapy solutions. At Careboo, a pioneering sleep health and advanced medical technology company, care is taken in serving as a premier MDR compliant breathing device manufactor China(https://www.careboohealth.com/product-category/snoring/) while prioritizing clinical safety. By adhering to the rigorous EU 2017/745 Medical Device Regulation and prioritizing clinical safety and regulatory alignment over standard sleep aids, Careboo provides…

How CE Approved Auto Ventilator Wholesale China Meets Rising Clinical Requiremen …
CE approved auto ventilator wholesale China(https://www.careboohealth.com/product-category/snoring/) has become an indispensable sourcing channel for hospitals, distributors, and healthcare providers looking for high-quality devices that balance safety with performance and scalability. Leading this transformation is Careboo, an healthcare technology company dedicated to improving sleep health through innovation, compliance, and patient-centric design.
Emergence of Clinical Demand for Automatic Ventilators
Since 2008, clinical requirements for automatic ventilators have steadily grown over time. Occlusive Sleep Apnea (OSA),…
More Releases for MDR
MDR Compliant Breathing Device Manufactor China vs Low-Cost Alternatives
Global healthcare standards have reached a crucial turning point, prompting an unprecedented need for high-performance respiratory therapy solutions. At Careboo, a pioneering sleep health and advanced medical technology company, care is taken in serving as a premier MDR compliant breathing device manufactor China(https://www.careboohealth.com/product-category/snoring/) while prioritizing clinical safety. By adhering to the rigorous EU 2017/745 Medical Device Regulation and prioritizing clinical safety and regulatory alignment over standard sleep aids, Careboo provides…
Growing EU Demand for MDR Certified CPAP Humidification Device Manufacturer
As European populations continue to struggle with sleep disorders such as obstructive sleep apnea (OSA), demand for advanced, compliant, and patient-centric respiratory therapy equipment is rising rapidly. Healthcare providers and distributors are increasingly turning to an MDR certified CPAP humidification device manufacturer(https://www.careboohealth.com/product-category/snoring/cpap-machines/) capable of meeting Europe's stringent regulatory, safety, and performance standards. In this context, Careboo has emerged as an invaluable healthcare technology partner, capitalizing on innovation, regulatory excellence, and…
MDR Certified CPAP Respiratory Device Manufacturer vs Standard CPAP Producers
As global healthcare regulations tighten and patient safety becomes the cornerstone of medical equipment production, certified excellence has never been more critical. Careboo is proud to stand as a pioneer of sleep health and pain management technology and proud to hold MDR certified CPAP respiratory device certification. Through advanced sleep monitoring combined with stringent clinical compliance measures, Careboo bridges the gap between basic respiratory assistance and high-tier medical therapy therapies…
IBN Technologies Advanced MDR Security Strengthen Enterprise Cyber Resilience
In an era where cyber threats evolve faster than traditional defenses, enterprises are rethinking their security operations to safeguard critical assets and ensure continuity. IBN Technologies has announced the launch of its advanced MDR security solutions(https://www.ibntech.com/managed-detection-response-services/), designed to deliver proactive, 24/7 protection across endpoints, cloud environments, and hybrid infrastructures. The service blends cutting-edge analytics, artificial intelligence, and expert human intelligence to identify and respond to cyber threats in real time.…
Managed Detection and Response (MDR) Market Overview (2024-2030)
Managed Detection and Response (MDR) Market is expected to reach USD 22.25 Bn by 2030, at a CAGR of 18.4% during the forecast period.
Managed Detection and Response (MDR) Market Overview:
The Managed Detection and Response (MDR) market has gained significant prominence in recent years as organizations across various sectors face increasingly sophisticated cyber threats. MDR services offer a comprehensive approach to cybersecurity by combining advanced threat detection technologies, continuous monitoring, and…
Managed Detection And Response Mdr Service Market Size Analysis by Application, …
USA, New Jersey- According to Market Research Intellect, the global Managed Detection And Response Mdr Service market in the Internet, Communication and Technology category is projected to witness significant growth from 2025 to 2032. Market dynamics, technological advancements, and evolving consumer demand are expected to drive expansion during this period.
The Managed Detection and Response (MDR) service market is expanding rapidly due to the rising number of cyber threats and the…
